Partial-thickness rotator cuff tears: a review of current literature on evaluation and management
Article information
Abstract
Rotator cuff disease is a common cause of shoulder pain for which partial-thickness rotator cuff tears occupy a significant proportion. Such tears are often difficult to diagnose and manage in the general clinic setting. A review of the available literature from well-known databases was performed in this study to provide a concise overview of partial-thickness rotator cuff tears to aid physicians in their understanding and management.
INTRODUCTION
Rotator cuff disease is a commonly encountered disorder of the shoulder and encompasses a wide spectrum of problems, including partial-thickness rotator cuff tear (PTRCT). The estimated prevalence of PTRCTs is 13% to 37% and is expected to increase with the increase of the aging population due to the positive correlation between age and rotator cuff disease [1]. Despite this burden on patients, in-depth data on the management of PTRCTs are relatively lacking in the literature relative to those available on full-thickness rotator cuff tears (FTRCTs) [2]. This review serves to discuss the often difficult diagnosis of PTRCTs as well as the current treatment modalities.
ANATOMY
The rotator cuff is composed of the subscapularis, supraspinatus, infraspinatus, and teres minor tendons. The subscapularis inserts onto the lesser tuberosity, while the other three muscles insert onto the greater tuberosity. There is significant interdigitation of these tendons, the shoulder capsule, and the coracohumeral ligament, which are in close relation to one another [3]. The “critical zone” of hypovascularity and histologically-correlated degeneration [4] is close to the insertion of the supraspinatus on the humerus. This “critical zone” is predominantly observed on the articular side and extends from the musculotendinous junction to within 5 mm of the insertion [5].
PTRCTs can be articular-sided, bursal-sided, intra-tendinous, or a combination. Based on the literature, articular-sided tears are two to three times more common than bursal-sided tears [6]. Most PTRCTs in older patients occur on the articular side of the supraspinatus tendon due to degenerative changes and ischemia at the “critical zone” of hypovascularity (insertion site), which worsen with age [6]. Therefore, tears on the articular side of the supraspinatus tendon are more common.
RISK FACTORS
Studies have shown that certain factors predispose individuals to PTRCTs. These include age, overhead activities causing increased load on the shoulder, smoking, obesity, and trauma [7-10]. Rotator cuff tears are mainly found in middle-aged and older patients as aging tendons undergo degeneration that can lead to microtears, calcification, and fibrovascular proliferation. Observational data show a linear increase in the occurrence of RCTs with age [11]. Trauma can also cause rotator cuff tears. A previous study revealed that 58% of patients presenting to the emergency department with acute shoulder trauma and normal radiographs who were unable to abduct above 90° exhibited acute traumatic tears of the rotator cuff [12].
PATHOGENESIS AND COURSE
Pathogenesis
The cause of PTRCTs is likely multifactorial, and degeneration, impingement, and overload are all contributors. These causes can be categorized as intrinsic and extrinsic [13]. Intrinsic causes refer to injuries arising within the tendon from degeneration, tendon overload, or other insults. Tensile overload during eccentric contraction with overhead activities is a common mechanism of injury for specific athletes and vocations. An avascular “critical zone” develops at the site of injury due to an intrinsically underdeveloped microvascular system, which reduces the potential for recovery [14,15].
Sports and occupations requiring overhead activity result in a high occurrence of rotator cuff tears [7]. Athletes performing overhead activities commonly injure their rotator cuff due to tensile overload during eccentric contraction. As an example, when a pitcher's throwing arm decelerates after release of the ball, the lengthening posterior rotator cuff muscles contract to slow the arm. This eccentric contraction places a large tensile load on the posterior rotator cuff [14-16].
Extrinsic causes refer to injuries caused by external impingement from compressive forces exerted by surrounding structures such as the acromion, coracoacromial ligaments, coracoid process, and acromioclavicular joint with osteoarthritic changes on its under surface. Glenohumeral instability can also lead to secondary compressive forces such as impingement of the rotator cuff during subluxation of the glenohumeral joint [14-16].
Tension overload from the distractive forces of throwing or trauma overpowers the ability of the rotator cuff to maintain the stability of the glenohumeral joint. Weakness of the rotator cuff muscle causes the glenohumeral joint to sublux, leading to internal impingement, which contributes to development of various pathologies such as articular-sided rotator cuff tears, impingement of the superior glenoid rim, and even labral pathology [8].
The pathogenesis of articular- and bursal-sided PTRCTs may differ due to differences in blood supply, biomechanical and histologic properties, associated changes of the acromion, and association with trauma. Studies have shown that intrinsic factors such as hypovascularity and decreased tensile strength resulted in articular-sided tears, while both intrinsic and extrinsic factors subjected the bursal side of the rotator cuff to greater wear [6]. Most often, rotator cuff lesions begin as partial tears of the under surface or articular portion of the supraspinatus tendon. Over time they can progress to FTRCTs to include the supraspinatus, infraspinatus, subscapularis, and biceps tendons.
Natural Course of Disease
A study reporting on 40 patients with articular-sided tears observed an increase of tear size in 21 patients (53%) and progression to full-thickness tears in 11 patients (28%) on repeat arthrography at a mean of 412 days, indicating that articular-sided tears usually worsen over time. The onset of pain or increase in pain, with or without accompanying weakness in active arm elevation, typically signals increasing size of cuff tears [17]. The limited healing potential of partial-thickness cuff tears is supported by histologic studies that have reported on the avascularity of the proximal stumps of the cuff with no signs of active repair [17]. Although it has been demonstrated that some patients do become asymptomatic over time, few heal anatomically.
Risk of Propagation of Tears
A study in 2022 evaluated 412 magnetic resonance imaging (MRI) studies from 206 patients with conservatively treated P- or FTRCP over 20 years. Among all the patients, 42% of PTRCTs progressed in size and 29% progressed to FTRCT. At 5 years, the rates of progression for 57% for partial-thickness tears were identified. Factors associated with tear progression included rotator cable integrity (P=0.001), subscapularis involvement (P=0.004), tear retraction (P<0.001), and tear width (P<0.001) [18]. Another study of 195 subjects with asymptomatic rotator cuff tears showed that patients with <50% tendon involvement exhibited a 14% chance of tear progression, while patients with >50% tendon involvement progressed 55% of the time [19].
CLINICAL PRESENTATION
Patients typically complain of pain that is more severe at night, especially when lying on the affected shoulder, and is localized to the lateral deltoid. Overhead activities such as lifting objects off a shelf or brushing one’s hair trigger the pain. Athletes performing repetitive overhead activity, for example swimmers or bodybuilders, may experience pain, weakness, or a decline in performance. In contrast to FTRCTs, PTRCTs result in greater stiffness, and non-physiologic tension on the remaining fibers may lead to more severe pain. A study reported significantly (P<0.01) higher levels of an afferent nerve pain mediator in the subacromial bursae of patients with PTRCTs compared to patients with FTRCTs. Higher levels of the pain mediators correlated with significantly (P<0.001) higher pain levels in the group of patients with PTRCTs [20].
Many patients experience a painful arc of motion between 60° and 120° of elevation with or without apparent or real muscle weakness. Crepitus, weakness, and positive impingement signs are other frequently observed physical signs [21]. Hawkins (passive internal rotation of the arm with 90° of flexion of the shoulder and elbow) and Neer’s (passive flexion and internal rotation of the shoulder) tests may be repeated after injection of 10 mL of 1% lidocaine into the subacromial space (impingement test). Reduction of pain on repeat testing after subacromial injection is indicative of rotator cuff inflammation.
Jobe’s sign (pain and reduced supraspinatus muscle strength on active resistance to shoulder abduction with the shoulder positioned in 90° of abduction) may also be positive in PTRCTs. The sulcus sign, the relocation test, and the degrees of anterior and posterior humeral translations, which are used to evaluate unidirectional or multidirectional shoulder instability, are recommended in young throwing athletes who may possess both shoulder instability and rotator cuff injury because the instability of the glenohumeral joint causes impingement of the rotator cuff during subluxation of the joint [13].
Patients with FTRCT may have near-complete resolution of pain, but continue to have loss of strength after a subacromial injection of 10 mL of 1% lidocaine. The maintenance of strength with reduction of pain suggests either rotator cuff inflammation or a PTRCT. The external-rotation lag sign had a specificity of 98% and a sensitivity of 56% in diagnosing FTRCTs [22]. However, there is limited evidence for use of external-rotation lag sign in diagnosing PTRCTs. Elderly patients older than 60 years have a 98% chance of rotator cuff tear if they present with two of the aforementioned findings [23].
DIAGNOSTIC IMAGING
X-Rays
Initial evaluation of a patient with shoulder pain and dysfunction should always include a complete set of plain radiographs of the shoulder in order to evaluate other causes of shoulder pain and to assess acromial morphology. However, these are rarely helpful in finalizing the specific diagnosis of a PTRCT. Some common findings of radiographs include the presence of a subchondral cyst in the greater tuberosity as well as the presence of a greater tuberosity “notch,” which was described in a study of 40 baseball players with articular-sided PTRCTs at the supraspinatus-infraspinatus interval, of which 38 exhibited this radiological finding [24]. A supraspinatus outlet view may be useful in visualizing bony structures of scapulohumeral motions such as bony spurs or ligamentous calcifications that may cause impingement of the underlying rotator cuff. An axillary view is helpful in excluding shoulder dislocation in trauma cases.
Arthrography
Studies have reported wide ranges of accuracy of 15% to 83% for arthrography [25]. Such imaging may have value in the diagnosis of FTRCT with the advantages of being relatively cheap and readily available. However, its role in evaluation of PTRCT is limited. A negative arthrogram obtained for evaluation of a painful shoulder cannot reliably rule out the presence of a PTRCT [26].
Bursography
Studies have also reported wide ranges of reported accuracy of 25% to 67% for bursography [27]. In addition to arthrography, bursography may be used to detect bursal-surface PTRCTs that are inaccessible to arthro-graphic dye. However, subacromial inflammation and adhesions limit the value of this technique. Considering these disparate data findings, arthrography and bursography have been largely replaced by ultrasonography (US) and MRI.
Ultrasonography
To identify PTRCTs using US, a study reported that “focal heterogeneous hypoechogenicity” points toward the presence of a PTRCT [28]. Fluid within one of the cuff surfaces or within the cuff substance produces a focal hypoechoic area. Linear echogenicity within the cuff substance with or without thinning of the cuff may also represent a PTRCT. A study reported preoperative US findings of a mixed hyperechoic/hypoechoic focus in the supraspinatus tendon to have a sensitivity of 93%, specificity of 94%, positive predictive value of 82%, and negative predictive value of 98% [29]. With its high accuracy rates, relatively low cost, and high degree of patient tolerance, US remains an attractive option for clinicians. However, its effectiveness may be limited by the number of trained personnel to perform and interpret its results, as evidenced by only a 41% detection rate of PTRCTs [30].
Magnetic Resonance Imaging
Diagnosis of PTRCT on MRI is suggested by increased signal in the rotator cuff without evidence of tendon discontinuity on T1-weighted imaging. A PTRCT is depicted as further signal increase on T2-weighted images with a focal defect that is intra-tendinous or limited to one surface and does not extend through the entire tendon. Rotator cuff tendinitis may also produce increased signals and loss of anatomic definition of the cuff on T1-weighted and proton-density images, similar to the appearance of a PTRCT. However, tendinitis is distinguished from PTRCT by the finding of only moderate or decreased signal on T2-weighted images [26].
Placement of the arm in a position of abduction and external rotation (ABER view) has also been a useful adjunct to routine imaging to identify not only articular-surface tears, but also labral lesions, especially in throwers, who often demonstrate this combined injury pattern [31]. With arthroscopic findings as the gold standard, a study found preoperative gadopentetate dimeglumine contrast magnetic resonance arthrography to have a sensitivity of 84%, specificity of 96%, positive predictive value of 93%, and overall accuracy of 91%, with coronal oblique T1 fat-suppressed images being most valuable [13]. US and MRI provide relatively similar accuracy rates for diagnosis of PTRCTs. However, a significant advantage of MRI is its ability to diagnose the concomitant pathologic lesions often seen with PTRCTs, including labral tears and biceps tendon lesions.
Arthroscopy
The gold standard for diagnosis and assessment of PTRCTs is arthroscopic inspection. Through direct inspection and probing of the tendon from its articular and bursal surfaces, a thorough examination of the cuff footprint may be performed. This improves the identification and treatment of these lesions. Several arthroscopic techniques have been described for intraoperative assessment and diagnosis of surface tears including tissue staining with methylene blue, suture marking, and the "bubble sign" for intra-tendinous tears [32]. Regardless of the technique used, arthroscopy provides the surgeon an opportunity to directly assess the quality of the rotator cuff tissue, as well as perform comprehensive, systematic diagnostic arthroscopy and bursoscopy.
Summary of Diagnostic Imaging
US and MRI have similar utilities in the diagnosis of PTRCTs. US can provide a cheaper and non-invasive alternative for evaluation of these tears, but it is highly operator-dependent and does not provide information regarding concomitant pathologies. In many patients presenting with vague signs and symptoms, MRI offers a comprehensive evaluation of the shoulder. For most patients with suspected PTRCT, especially young and overhead throwing athletes, magnetic resonance arthrography is the best choice to visualize these tears and to assess concomitant pathology [6]. However, given the high proportion of the population with asymptomatic PTRCT, MRI should be considered in conjunction with clinical evaluation.
CLASSIFICATION
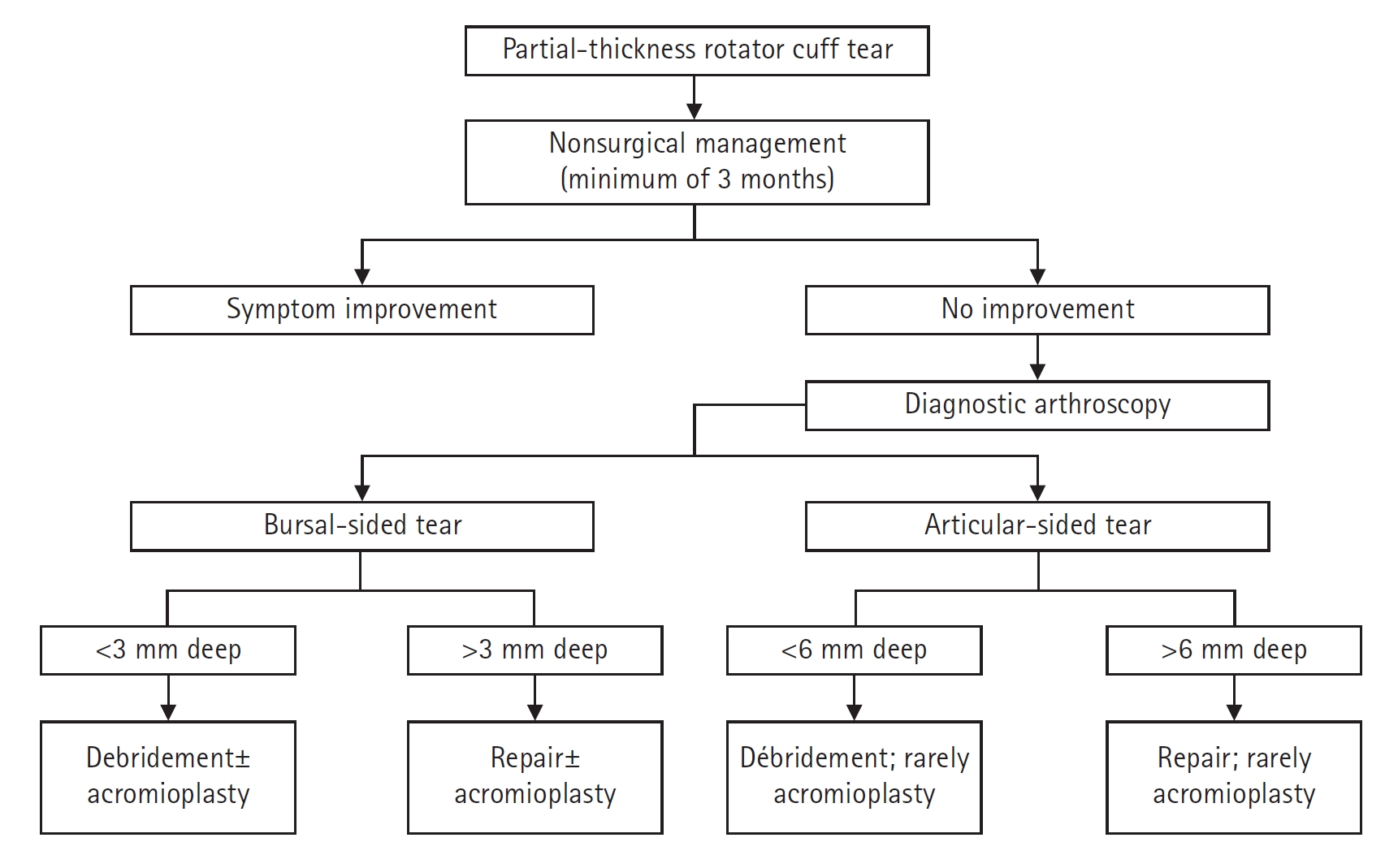
PTRCTs are commonly classified into three main categories based on location, size of the tear, and tendons involved. The Ellman classification is based on location (articular, bursal, and intra-tendinous) and grade of tear. Grade I tears have a depth less than 3 mm. A tear of depth 3 to 6 mm is classified as grade II. A grade III tear involves more than 50% of the cuff thickness [26,33]. A study showed that the superior-to-inferior insertion width of the supraspinatus tendon averages 12.7 mm to 12.1 mm. Therefore, an articular-sided partial-thickness tear of the supraspinatus tendon of a length greater than 7 mm is considered greater than 50% of the tendon thickness [2].
Despite its widespread use, the classification ignores a number of important aspects including tissue quality, area of the tear (i.e., anterior-posterior vs. medial-lateral), and cause of the tear. It is necessary to accurately define the tear etiology in order to determine the most appropriate treatment plan. Furthermore, there is relatively poor inter-observer reliability of this classification system when using imaging modalities or even dedicated arthroscopic videos.
CONSERVATIVE MANAGEMENT
The optimal treatment of PTRCTs is influenced by multiple factors, including patient age, symptoms, functional deficit, size of the tear, tear location, nature of onset (e.g., degenerative versus traumatic), etiology, vocation, and daily activities. In the majority of cases, a trial of conservative treatment (e.g., activity modification with avoidance of overhead or pain-provoking activities, non-steroidal inflammatory drugs (NSAIDs), pain medications, physiotherapy, and steroid injection) is reasonable since, unlike FTRCTs, the risk of fatty infiltration, muscular atrophy, and significant tear progression is relatively minimal.
Denkers et al. [34] conducted a study where 38 of 76 consecutive patients with PTRCTs were treated conservatively and followed. At an average of 4 years, 91% of the patients were satisfied with the conservative management. Patients who had an atraumatic tear that involved <50% of the tendon thickness on the non-dominant hand were more likely to be treated conservatively [35]. Even in certain athletic populations, conservative treatment of PTRCTs may be preferred. In the throwing athlete, due to the downtime and the negative side effects such as stiffness and decreased range of motion associated with surgery, athletes with tears involving up to 75% of the tendon thickness may be treated conservatively [35]. Although there is a paucity of reliable reports on the clinical outcome of conservative treatment of PTRCTs, most patients improve with conservative measures over 6 months, while some continue to improve for up to 18 months [6]. However, one should note the potential risk of tear progression when opting for conservative management.
Physiotherapy
In PTRCTs that are left unaddressed, scapulothoracic dyskinesia due to pain increases the likelihood of extrinsic acromial impingement on the rotator cuff. As soon as the inflammation and pain subside, a dedicated physiotherapy program should be first aimed at eliminating capsular contractures and regaining full motion. As motion improves, attention should then be focused on strengthening the rotator cuff and periscapular musculature. The function of the rotator cuff in dynamic stabilization of the glenohumeral joint is maximized through progressive, resistive exercises involving the use of elastic bands or free weights.
Rehabilitation of the periscapular musculature may serve to restore normal scapulothoracic mechanics and to minimize dynamic impingement secondary to scapulothoracic dyskinesis. Conservative management of rotator cuff injuries should include a comprehensive rehabilitation program. Wilk et al. [36] have extensively reported on shoulder rehabilitation for the throwing athlete with many of the same principles applied to all types of athletes with varying patterns of rotator cuff injury. A recent review by Edwards et al. [37] provided an evidence-based 4-phase exercise protocol for conservative management of rotator cuff injury.
Early rehabilitation should focus on reducing pain and inflammation and restoring normal range of motion. Local modalities like ice, electrical stimulation, and manual therapies can be utilized. Avoidance of activities like weightlifting that could worsen the pain should also be practiced. The intermediate phase should include progressive strengthening of the scapular musculature as well as the rotator cuff. Emphasis should be placed on stretching of contracted posterior capsular tissues, which have been shown to result in loss of internal rotation. Eccentric and plyometric strengthening of the rotator cuff should be included in the rehabilitation program to mimic the deceleration and follow-through phases of the throwing motion [38].
Pharmacological Management
NSAIDs are useful in reducing the associated pain and inflammation. Corticosteroids are another option for symptomatic relief of PTRCTs. Although reports have questioned the efficacy of these injections, they have been found to be useful adjuncts. Depending on the location of the tear, subacromial or intra-articular corticosteroid injections can be used for patients with persistent symptoms refractory to NSAIDs.
Subacromial injection is more useful for bursal-sided PTRCTs than articular-sided PTRCTs. On the other hand, intra-articular injections have been shown to be more effective for patients with articular-sided PTRCTs [39]. The use of corticosteroid injections for acute relief of pain can be beneficial for allowing a more aggressive initiation of directed physical therapy. Therefore, individual patient responses to the injections should guide the treatment approach. However, no more than two to three injections are recommended because of the potentially deleterious effects on rotator cuff tissue, especially in younger athletes [13].
Polydeoxyribonucleotide (PDRN) is an emerging treatment that has been found to significantly reduce musculoskeletal pain associated with rotator cuff tendinopathy [40]. PDRN is a compound formed by deoxyribonucleotide polymers of varying lengths of base pairs and nucleosides derived from salmon sperm [41]. In addition, PDRN is known to promote the regeneration of tendon or ligament injuries in animal models [42].
Atelocollagen is a collagen treated with proteolytic enzymes to remove the terminal telopeptides with low immunogenicity. The use of atelocollagen injections in the torn tendon greatly increased healing in patients with PTRCTs, as seen by the reduced tear sizes. It has also been shown to improve functional outcomes at final follow-ups compared to those who did not receive an injection [43]. However, there is currently only evidence for the use of atecollagen injections for intratendinous rotator cuff tears.
SURGICAL MANAGEMENT
Surgical intervention can be considered for patients with symptoms of sufficient duration and intensity after conservative treatment fails. Histologic studies have shown that PTRCTs have essentially no ability to heal themselves over time. Tears biopsied at the time of operative intervention exhibited granulation tissue with rounded, avascular tissue margins without evidence of healing. Another study followed 40 articular-sided tears over 2 years with arthrography and showed 80% tear progression (28% to full thickness) [17].
According to the literature, the timing of surgery ranges from a few months to 1.5 years from onset, but it should be based on patient symptoms, improvement, rate of improvement with conservative management, functional demands, and comorbidities as well as the goals of the patient [6]. In young and active patients, restoring strength and function should be a priority, while reducing surgical risk and providing pain relief should be the focus for older patients. The onset of acute, posttraumatic weakness in young or physically active patients is typically a strong indicator for surgical management.
Although surgical management is also recommended for patients with tears extending beyond 50% tendon thickness, an important factor in this decision is patient symptoms. Twenty asymptomatic overhead athletes who were evaluated with MRI exhibited up to 40% full or partial thickness tears on the dominant shoulders without any subjective symptoms or requiring any treatment after 5 years [44]. Therefore, decisions for surgical management should not be solely based on MRI findings. Many surgical procedures have been recommended for treatment of PTRCTs. These include debridement of tears with or without acromioplasty and both open and arthroscopic repair with or without acromioplasty.
Debridement of Tears without Acromioplasty
One study reported on a series of 79 shoulders treated with arthroscopic debridement and followed for a minimum of 25 months. Using the University of California, Los Angeles (UCLA) scores, 89% of the shoulders exhibited good or excellent outcomes at less than 5 years, with 81% of the shoulders maintaining that score at more than 5 years of follow-up [45]. Another study reported that approximately half of 57 patients with partial-thickness tears had successful results with arthroscopic debridement without acromioplasty at a minimum of 1 year after surgery. Other authors have reported success rates of 50% to 89% after arthroscopic debridement without acromioplasty [46].
Debridement of Tears with Acromioplasty
In a study, 37 patients with tears involving less than 50% of the cuff thickness (Ellman grade 1 and 2) were treated with debridement and subacromial decompression. Twenty-four patients had articular-sided tears, whereas 13 had bursal-sided tears. The study noted that bursal-sided tears fared significantly better with respect to pain score and function (P<0.05 for both) at 6 months, but that the groups were not significantly different at 1- and 2-year follow-ups [47].
Another 105 patients with Ellman grade 1 or 2 tear were treated with debridement and subacromial decompression and followed for 2 to 10 years. This time, the articular-sided PTRCTs fared better, with a 3% failure rate over the time of the study compared with a 29% failure rate for bursal-sided PTRCTs [48]. A third study followed 26 patients for a minimum of 5 years after surgery with physical examination and ultrasound evaluation. They reported further rotator cuff disease progression after debridement and acromioplasty for Ellman grade 2 PTRCTs. They also noted that the final constant scores was significantly lower for bursal-sided tears compared to articular-sided tears, with scores of 61.5 and 72, respectively. In addition, nine shoulders progressed to FTRCTs [49]. Patients with concomitant diseases such as subtle instability, acromioclavicular joint arthritis, adhesive capsulitis, or glenohumeral osteoarthritis appear to exhibit poorer outcomes after debridement of PTRCTs and acromioplasty.
Repair of Tears
One hundred patients with PTRCT who underwent arthroscopic trans-tendinous repairs exhibited significant improvements in UCLA, Simple Shoulder Test, and visual analog scale scores. Ninety-six cases were rated good or better according to the UCLA score. No significant differences in range of motion were noted compared to the contra-lateral side at the 24-month follow-up. Arthroscopic trans-tendon repair of partial articular-sided rotator tendon avulsions (PASTA repair) is an effective procedure that leads to significant improvement in pain and shoulder function with high patient satisfaction rates and low complication rates [50].
In 2017, Osti et al. [51] evaluated 18 studies published between 2005 and 2016 describing in situ repairs of PASTAs. They revealed good and excellent results with low complication rates in most studies. In 2016, Ranalletta et al. [52] evaluated 80 patients who had undergone arthroscopic in situ PTRCT repairs with a minimum of 2-year follow-up. The authors observed significant functional improvements and pain relief in most patients, achieving 92% satisfaction with a low rate of complications in the midterm follow-up. Forty-two consecutive shoulders with greater than 50% PTRCT were treated with arthroscopic repair after purposeful conversion to FTRCTs. At final follow-up of an average of 11 months, 37 of the 42 shoulders (88%) exhibited an intact rotator cuff on ultrasound with improved American Shoulder and Elbow Surgeons scores and a 93% patient satisfaction rate [53].
Many studies have compared the effectiveness of arthroscopic in situ repairs against arthroscopic completion of the tear and subsequent formal repair. In 2013, Franceschi et al. [54] prospectively carried out a study comparing the two groups and determined that the results were comparable in terms of functional outcomes and repair failure rates. In 2015, Castagna et al. [55] and Shin et al. [56] carried out a similar study and concluded that the techniques did not exhibit any difference in terms of function and pain between the two groups.
CONCLUSIONS
PTRCT is a common condition with symptoms that can be debilitating. Diagnosis may be difficult, and abnormal findings on imaging may be observed even in asymptomatic patients. The pathogenesis of PTRCTs involves more frequent intrinsic than extrinsic degeneration, suggesting that conservative treatment focused at restoring or maintaining normal shoulder dynamics should be attempted first. However, conservative treatment, particularly in the short term, must be balanced against the potential for long-term anatomic disease progression. Patients who do not respond well to conservative treatment modalities can be considered for surgery, taking into account the severity of their symptoms. For the average symptomatic person, surgery can provide excellent pain relief and return of function.
Notes
Author contributions
Conceptualization: AHCT. Resources: RR. Software: AHCT. Supervision: AHCT. Validation: AHCT. Visualization: AHCT. Writing-original draft: RR, JG. Writing-review & editing: RR, JG, AHCT.
Conflict of interest
None.
Funding
None.
Data availability
None.
Acknowledgments
None.