Patient-specific implants in reverse shoulder arthroplasty
Article information
Abstract
Reverse total shoulder arthroplasty (RTSA) is widely popular among shoulder surgeons and patients, and its prevalence has increased dramatically in recent years. With this increased use, the indicated pathologies associated with RTSA are more likely to be encountered, and challenging patient presentations are more likely to be seen. One prominent challenging presentation is RTSA patients with severe glenoid bone loss. Several techniques with varying degrees of invasiveness, including excessive reaming, alternate centerline, bone grafting, and patient-specific implants (PSIs), have been developed to treat patients with this presentation. PSI treatment uses a three-dimensional reconstruction of a computed tomography scan to design a prosthetic implant or component customized to the patient’s glenoid morphology, allowing compensation for any significant bone loss. The novelty of this technology implies a paucity of available literature, and although many studies show that PSIs have good potential for solving challenging shoulder problems, some studies have reported questionable and equivocal outcomes. Additional research is needed to explore the indications, outcomes, techniques, and cost-efficiency of this technology to help establish its role in current treatment guidelines and strategies.
INTRODUCTION
Total shoulder arthroplasty has become more common in recent years because of its high success with excellent outcomes [1]. Reverse total shoulder arthroplasty (RTSA) reverses the ball-and-socket shoulder joint and medializes the center of the joint to enhance the function of the deltoid muscle and increase stability in rotator cuff-deficient shoulders in a semi-constrained way [2]. Since the introduction of RTSA in 1985 by Paul Grammont and its approval by the Food and Drug Administration in 2004, it has gained a massive boost in popularity, which has contributed to an increase in the incidence of shoulder arthroplasty procedures [1]. Recent studies have confirmed that the rate of RTSA tripled between 2012 and 2017, and that rate is projected to continue increasing [3]. The mean age for people undergoing RTSA has decreased, with notable RTSA procedures being conducted for patients in the 50–64 age group [3]. The indications for RTSA are no longer limited to rotator cuff arthropathy but have expanded to include glenohumeral osteoarthritis (OA), acute fractures, inflammatory arthritis, humeral bone loss, pseudoparalysis, and revision of previous arthroplasties [1,3].
Reversal of the normal shoulder joint, however, disrupts the natural anatomy and has potential for a high complication rate [4]. A recent study with a large population reported that RTSA has a complication rate of 16.1% [5]. Some of the most frequent complications are scapular notching, acromial fracture, instability, component loosening (humeral and glenoid), infection, and neurologic injury [2,4,6,7]. Glenoid component mispositioning is a common and serious complication of RTSA that requires special consideration, even in seemingly straightforward cases [8]. An improperly placed component is associated with increased risk of dislocation, wear and loosening, and need for revision surgery [8]. Several techniques have been developed to simplify the cumbersome process of accurately positioning the glenoid component. Patient-specific implants (PSIs) are one such technique that has gained popularity in the past few years, but they have yet to be adopted as a standard of care in RTSA. PSIs involve the use of guides custom-made for each patient’s anatomy using three-dimensional (3D) reconstructions of computed tomography (CT) scans. Studies have confirmed that using a PSI improved the positioning of the glenoid component compared with standard instrumentation [9].
Understanding the indications, advantages, disadvantages, and clinical applicability of the PSI technique is pivotal for establishing its place in treatment guidelines and strategies. Therefore, our aim in this paper is to provide an up-to-date review of PSIs and offer proper recommendations and future expectations for their use.
GLENOID BONE LOSS
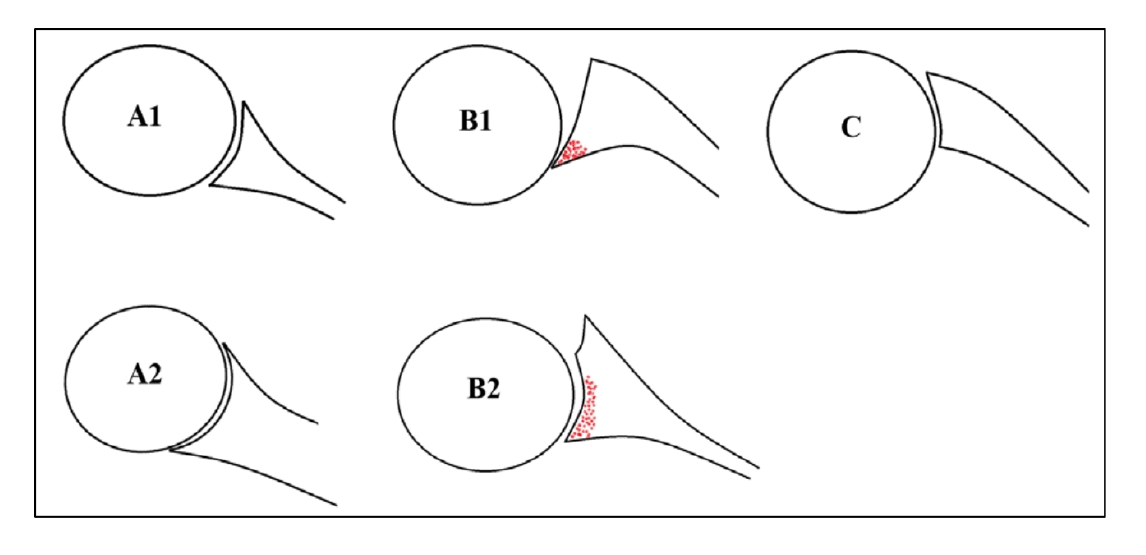
Several studies have described the different morphological features of the glenoid in people undergoing shoulder arthroplasty and the effects of those features on prognostic outcomes [10-13]. Frankle et al. [10] reported a 38% incidence of glenoid defects in patients undergoing RTSA. Several etiologies for glenoid bone loss have been described, including traumatic injuries, recurrent dislocations, cuff tear arthropathy, inflammatory arthritis, revision arthroplasty, and most notably, primary OA. The incidence of altered glenoid morphology in OA is approximately 40% [11]. The most common morphologic changes in OA occur predominantly in the horizontal plane, usually involving posterior humeral head subluxation with possible associated posterior glenoid bone loss [13]. This defect can range from minimal bone loss that can be treated with reaming to complex bone loss that can require corticocancellous bone grafting [14]. Different classifications have been used to describe patterns of glenoid bone loss. For horizontal plane morphology, the most common classification system was developed by Walch et al. [12]. In their classification, the glenoid morphology is categorized into three types (A, B, or C) based on CT findings (Fig. 1) [12]. In type A, the humeral head is centered in the glenoid with equally distributed forces across the whole glenoid, with minimal erosion in type A1 and more severe central bone loss with central cupula formation in type A2. In type B, the humeral head is subluxed posteriorly, with an asymmetric distribution of load; type B1 shows posterior joint space narrowing, subchondral sclerosis, and osteophyte formation, and type B2 shows posterior cupula formation that produces a biconcave morphology. In type C, the retroversion is greater than 25° regardless of erosion [12].
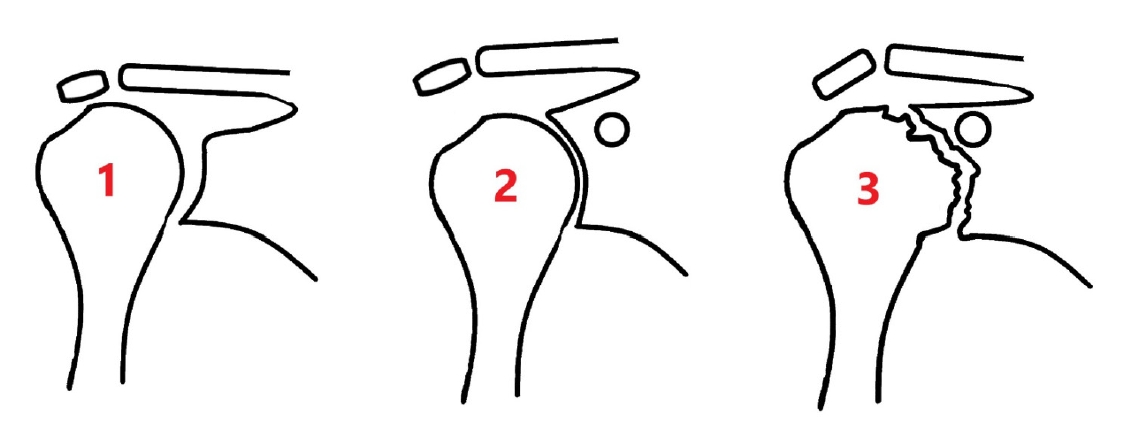
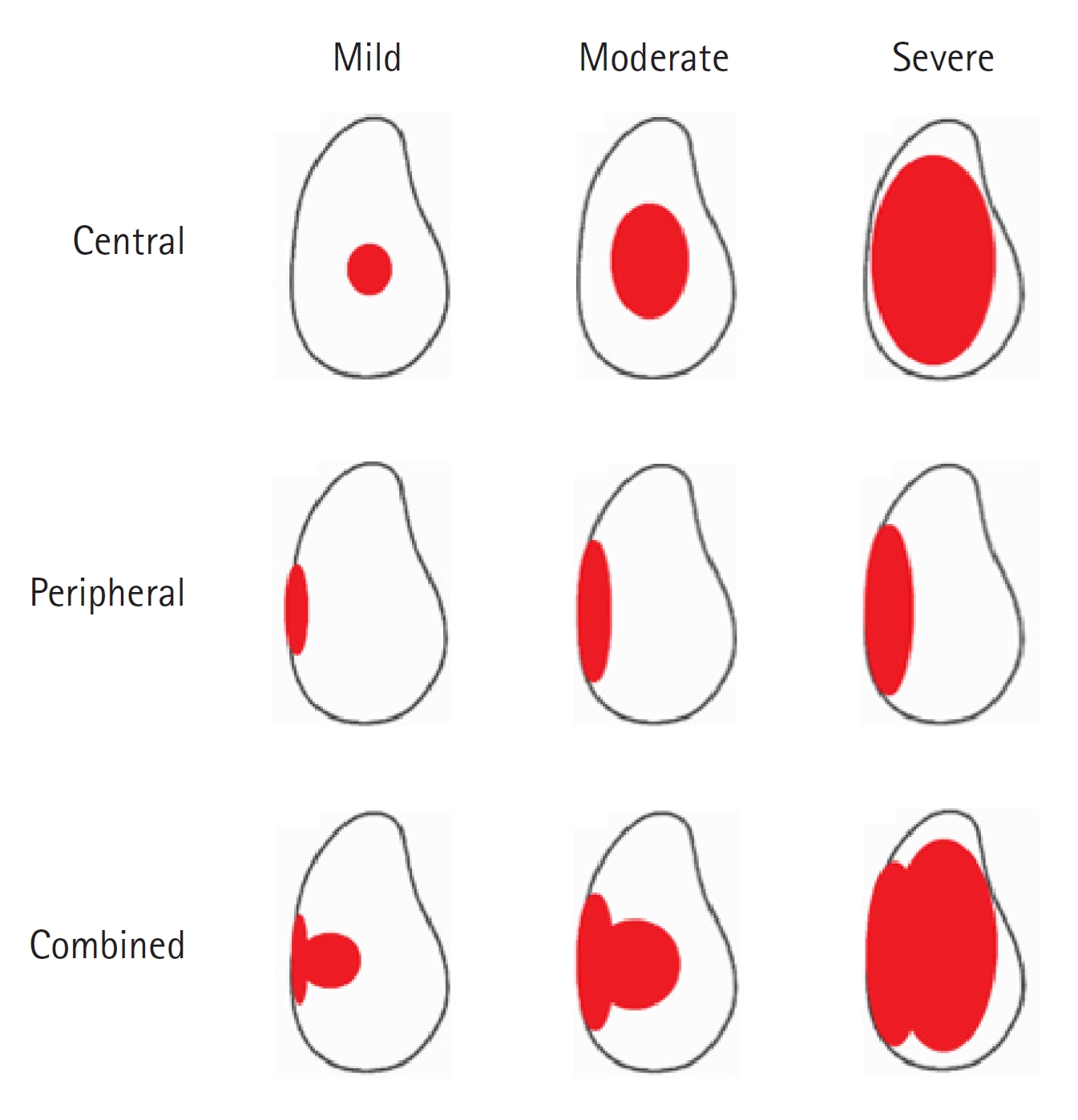
The Favard classification was developed to describe vertical plane glenoid morphology (Fig. 2) [15], and Lévigne and Franceschi [16] developed a classification system for central wear with medialization of the glenoid in patients with rheumatoid arthritis (Fig. 3). Antuna et al. [17] developed a classification system for glenoid bone loss in revision arthroplasty, and that system was later modified by Williams and Iannotti [18] for use in both primary and revision surgeries (Fig. 4). All these classification systems reflect the significance and implications of glenoid bone loss for prognostic outcomes of managed patients.
The depiction of stages of glenoid erosion in the setting of rheumatoid arthritis, according to the Levine classification.
The depiction of glenoid erosion following removal of the glenoid component, according to the Williams and Iannotti classification.
The placement of glenoid components, in terms of both fixation and position, is one of the most important factors in successful shoulder arthroplasty [13]. Glenoid bone loss, as described earlier, is one of the most important challenges in shoulder arthroplasty [7,13,14,19], and the literature shows that it is associated with worse outcomes and a higher complication rate [11]. Early loosening, in particular, can result from decreased bone stock, as well as poor fixation and mispositioning, which can result in rocking horse loosening [20]. Shapiro et al. [21] demonstrated that retroversion >15° is associated with decreased glenohumeral contact and subsequent increased focal pressure, as well as changes in forces that lead to increased posterior tension on the infraspinatus and teres minor tendons. A laboratory study by Martin et al. [22] showed that baseplate micromotion was significantly higher in glenoid models with 50% bone loss than in those with 25%, 10%, and no defects. A finite element analysis by Farron et al. [23] showed that a retroversion >10° is associated with a maximal micromotion increase of 706% and a mean micromotion increase of 669% at the cement–bone interface, as well as a 326% increase in cement stress at 20° of retroversion . It is of paramount importance to identify and describe bone loss preoperatively so that the surgical approach and technique can be tailored to fit the patient [11].
RTSA FOR GLENOID BONE LOSS
Since its introduction in 1985 by Paul Grammont, the ball-and-socket design of shoulder treatment, termed RTSA, has gained great popularity [24]. The four key principles of RTSA are: (1) medializing the center of rotation to decrease torque and subsequent loosening, (2) lowering the humerus to increase deltoid tension and compensate for deficient rotator cuffs, (3) using a fixed, distalized, and medialized center of rotation with respect to the joint line to provide inherent stability, and (4) using a large glenosphere to allow for a semi-constrained implant design with a proper range of motion [25-28]. While respecting those basic principles, the RTSA has undergone several modifications, and the indications have increased to include a multitude of pathologies [24,28]. Despite the use of different prosthetic designs, the basic components of RTSA remain unchanged: (1) a glenoid baseplate, (2) a hemispherical-shaped glenosphere, (3) a humeral stem or stemless component with a modular metaphyseal implant, (4) and articular polyethylene [28]. RTSA has become central to treating shoulders with severe glenoid bone loss because it offers a more robust design and better inherent stability than other arthroplasty options [24,27].
When faced with medialization due to bone loss, the ability to lateralize the glenoid component is crucial. In fact, Keener et al. [29] found lateralization to be the single most important factor in stability and proper range of motion. To address glenoid bone loss and enhance patient outcomes in RTSA, several surgical techniques have been developed. One of the most common approaches to glenoid bone loss and disturbed morphology is eccentric reaming, which has shown good results [30]. However, excessive reaming can further decrease the available bone stock and cause a loss of stability due to medialization of the glenoid [30]. Another technique is the use of an alternative centerline for central screw insertion. The standard centerline is at the center of the glenoid and is perpendicular to the surface, whereas the alternative centerline is at the center of the glenoid and is in line with the scapular spine [10,11]. This technique prioritizes baseplate fixation over anatomical positioning, with possible increase in scapular fractures and instability due to anteversion [31,32]. Another method for dealing with glenoid bone loss is bone grafting. Several techniques for glenoid bone grafting have been described, such as impaction grafting, cylindrical grafting, L-shaped grafting, U-shaped grafting, and grafting with internal fixation, with each technique suitable for a specific pattern of bone loss [20,33]. However, bone grafting has been associated with an increase in complication rates, including those for scapular notching, infection, and early loosening [34]. Malhas et al. [35] noted an overall complication rate of 31% and a revision rate of 16% in patients treated with autologous bone grafts and metal-backed glenoid baseplates. Neyton et al. [36] showed that RTSA with glenoid bone grafting resulted in good pain relief but low functional results after 2 years and was associated with scapular notching. PSI is another method that has been described to address severe glenoid bone loss. PSI involves the use of 3D-printed, custom-made glenoid components (Fig. 5) and was inspired by the successful use of custom acetabular cups in total hip arthroplasty [37]. PSI requires preoperative CT of the shoulder and 3D reconstruction to tailor the baseplate according to bone loss, but it enables individualization of the glenoid component to ensure proper fixation and sufficient plate–bone contact [38].
PATIENT-SPECIFIC IMPLANTS
Subsection 1: Indications and Benefits
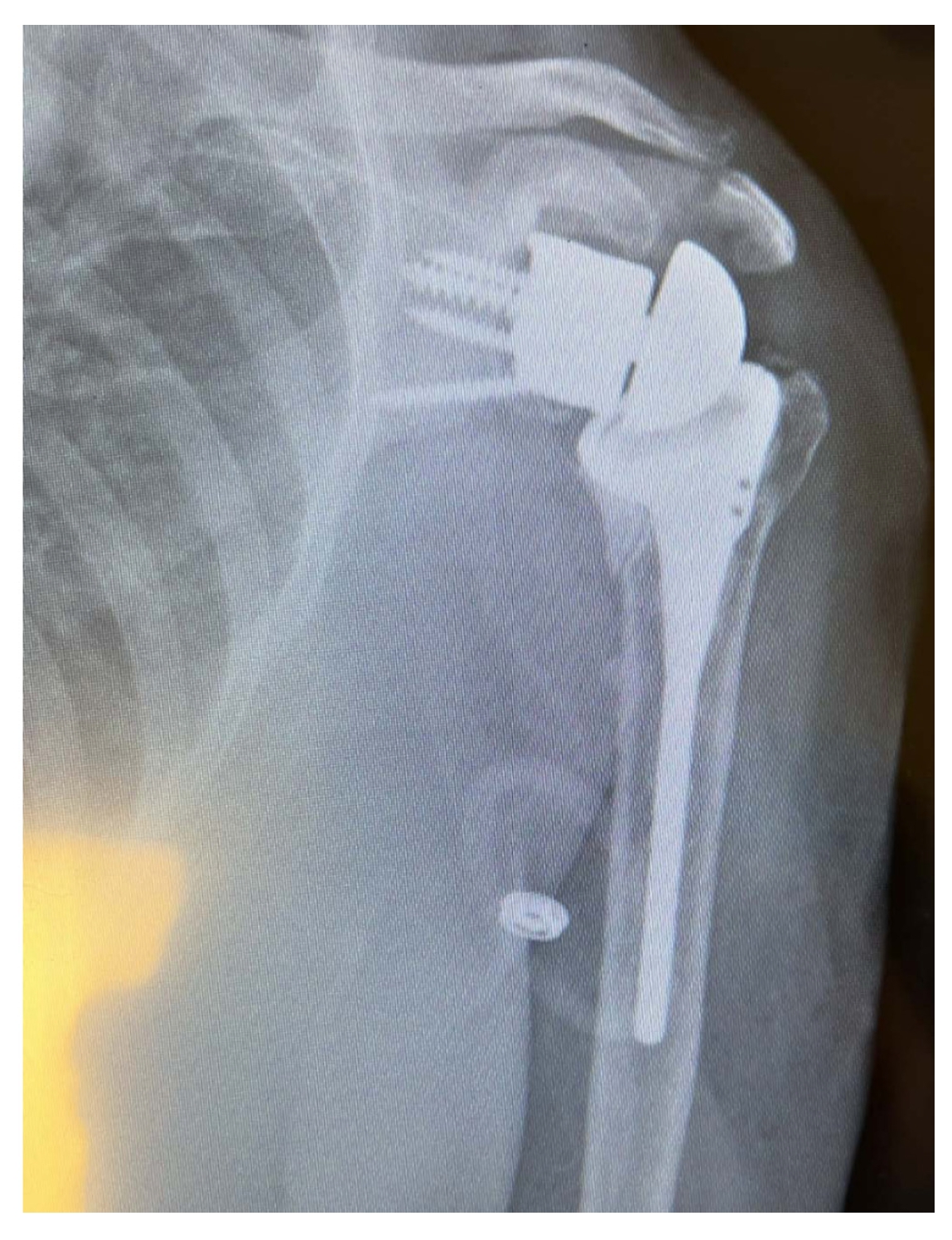
Custom-made glenoid implants provide an interesting solution for shoulders with glenoid bone loss, especially in advanced and severe cases in which a perfect fit is crucial to prosthesis survival [39]. Computer-assisted design/computer-assisted manufacturing (CAD/CAM) is used with statistical shape modeling and 3D CT reconstructions to appropriately replace the lost bone and reconstruct the joint line [40]. This enhances preoperative planning by simplifying proper positioning and enabling a better assessment of screw fixation [41]. Intraoperatively, proper exposure of the whole remaining glenoid should be obtained to assess the seating of the custom component on the bone. If any defect is noted, the bone should be contoured to ensure that the prosthesis is flush with the remaining bone [37]. A minimum vault depth of 10 mm should be obtained for the initial fixation, with sufficient volume for insertion of two peripheral screws (Fig. 6); achieving a minimum 50% insertion of the peg into the glenoid bone will lead to increased stability [35]. A central boss can also be used in cases with sufficient bone stock, further increasing the stability of the construct [38].
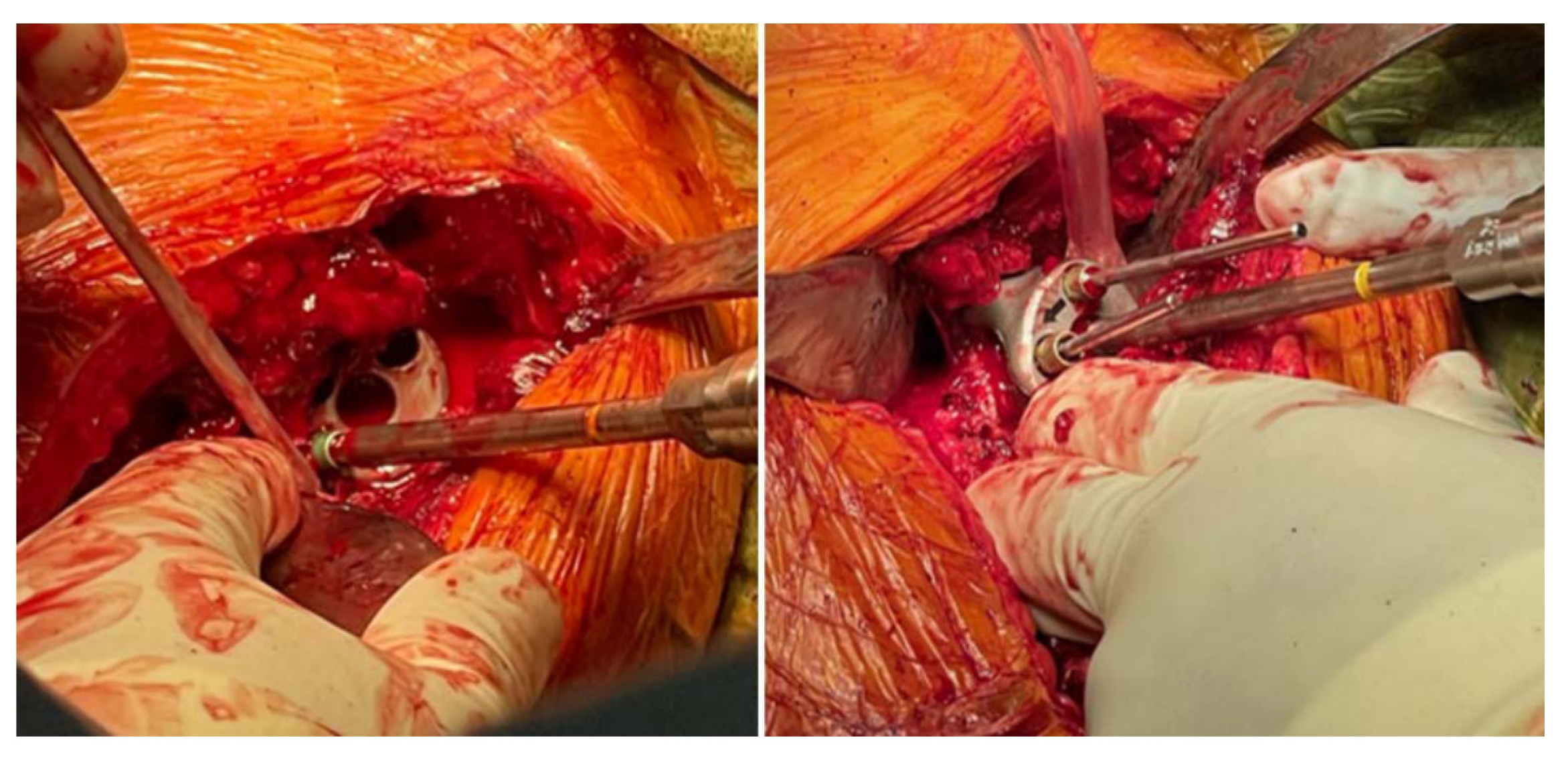
Intraoperative image detailing the fixation of a patient-specific implant using peripheral screws according to previously determined trajectories.
The decision to use a custom-made glenoid component is based on the functional needs of the patient and the severity of the bone defect [39]. The use of PSI is generally restricted to severe glenoid bone loss that is deemed unrepairable (or poorly repairable) by other surgical techniques [42-44]. As discussed earlier, the main advantage of PSI is its ability to accurately predict the degree of bone loss and to use a component that fits precisely, enabling proper restoration of the anatomic joint line (Fig. 7) [39,41]. Another benefit is the preoperative evaluation, which aids in screw direction, length, and proper positioning, due to the implant guides [41]. Customized surgery can also enhance joint stability by predetermining the use of intact elements, such as the spine of the scapula or the coracoid process [39].
Subsection 2: Clinical Results
Due to the increasing prevalence of RTSA, severe bone loss is becoming an increasingly encountered problem, especially following component loosening and in revision of primary arthroplasty [44]. Because the use of PSI in RTSA is a new concept extrapolated from hip surgery, clinical results are scant, and it is challenging to compare findings because little peer-reviewed research has reported the functional and radiologic outcomes of patients treated with a patient-specific glenoid component [43].
The use of a CAD/CAM hip-inspired implant (Stanmore Implants) for failed RTSA was described by Uri et al. [45] in 2014. They explored the outcomes of 11 patients with a mean follow-up length of 35 months. Their implant, which was inspired by restricted hip arthroplasty, was designed to facilitate glenoid fixation by attaching a large glenoid shell to the scapula rather than to the glenoid itself. Significant pain relief following surgery was noted: on a scale from 0 to 10, pain decreased from 5.6 (at rest) and 7.4 (during activity) to 1.1 and 2.1, respectively. Functional outcomes were also noted, with improvement of the Oxford shoulder score (OSS) from 47 to 31 and of the subjective shoulder value (SSV) from 22% to 45%. No glenoid loosening was observed; however, four patients needed further surgeries unrelated to the glenoid component.
Another study from Uri et al. [46] in 2014 detailed the short-term results of 21 patients who underwent revision arthroplasty using a hip-inspired CAD/CAM implant (Stanmore Implants) after experiencing posttraumatic humeral head replacement failure with associated glenoid deficiency. The functional results and pain management of the patients in that sample both improved; however, postoperative range of motion did not improve significantly. That study also revealed high complication rates, with 9 of the 21 patients suffering infections, prosthetic dislocations, periprosthetic fractures, or broken fixation screws.
A case report in 2014 by Berger et al. [47] discussed the use of a custom “patient matched-implant” glenoid component (Biomet) in a case of RTSA for severe glenoid deficiency. The patient showed improvement in range of motion, functionality, and pain sensation 10 months postoperatively. In 2017, Chammaa et al. [48] conducted a trial using custom hip-inspired TSA. They studied the results of 37 patients who underwent primary shoulder arthroplasty using CAD/CAM TSA due to severe glenoid bone loss and rotator cuff deficiency. The custom implant was hip-inspired and consisted of a large acetabulum-like glenoid shell fixed around the scapula. At the 5-year follow-up, the patients reported statistically significant improvements in pain, functional outcome scores, and range of motion. The mean revised OSS significantly increased from 11 to 27 points, and the SSV increased from 23% to 60%. However, the average postoperative forward elevation was only 64°, which the authors attributed to the implant's constrained design. During the 5-year follow-up period, reoperations were required in 9 of the 37 patients (24%), 6 of which (16%) were due to component-related complications—including aseptic loosening, fractures, and implant dislocation. Only one subject experienced glenoid loosening following a mechanical fall.
A study in 2019 by Debeer et al. [43] considered 10 patients with severe glenoid deficiency who underwent RTSA (primary or revision) using the Glenius Glenoid Reconstruction System (GGRS; Materialise). In terms of design, the GGRS is similar to the Comprehensive Vault Reconstruction System (VRS) but with addition of a custom glenosphere. The postoperative scores at an average follow-up of 30.5 months were as follows: visual analog scale (VAS) pain score of 3.3±2.5; Constant score of 41.3±17.5; Quick Disabilities of the Arm, Shoulder, and Hand (DASH) score of 35.8±18.4; and simple shoulder test (SST) score of 47.5±25.31. Two of the 10 patients (20%) experienced complications; one was related to instability and was treated with a larger polyethylene insert, and the other was related to a brachial plexus injury that partially recovered but left residual limitations in the range of motion. Radiologic evaluations of differences between planned and postoperative inclination and version were also performed in that study, and the authors found reliable correction of inclination but higher variability in version (4°±4° and 6°±4°, respectively). Those findings were explained by an inability to obtain adequate exposure, the bulkiness of the GGRS and its guide in larger defects, inadequate removal of loose fragments, or the time between the preoperative CT scan and the actual procedure.
Dines et al. [42] presented an article in 2017 about the use of the Comprehensive VRS (Zimmer Biomet) in two patients with severe glenoid bone loss. Those patients showed good clinical and range of motion outcomes, but a longer follow-up is required.
Rangarajan et al. [37] performed a single-center trial in 2020 on 18 patients undergoing primary and revision RTSA using the Comprehensive VRS (Zimmer Biomet). When comparing preoperative and postoperative clinical scores, the authors reported significant improvements after an average follow-up of 18.2 months: the DASH score improved from 57.4±16.5 to 29.4±19.5, the Constant score from 24.6±10.2 to 60.4±14.5, the American Shoulder and Elbow Surgeons (ASES) score from 32±18.2 to 79±15.6, the SST score from 4.5±2.6 to 9.3±1.8, the Single Assessment Numeric Evaluation (SANE) score from 25.4±13.7 to 72.2±17.8, and the VAS pain score from 6.2±2.9 to 0.7±1.3. Additionally, improvement was noted in forward flexion and abduction but not external rotation. No evidence of implant loosening or hardware failure was seen on radiographic evaluations. Despite those results, complications occurred in 21% of the patients.
Similarly, Bodendorfer et al. [38] reported short-term outcomes for RTSA using the VRS system for advanced glenoid bone loss or revision surgery in 12 shoulders. At an average follow-up of 30 months, all patients experienced significant pain relief and improvement in both functional outcomes and ROM (forward elevation, external rotation, and internal rotation): the median Numeric Pain Rating Scale score improved by 7 points, the SANE score by 43%, the ASES score by 45 points, and the Penn shoulder score by 49 points. Radiographic evaluation at the final follow-up showed that all implants were stable without any signs of loosening. No complications were encountered in any of the RTSA implants in that study.
In 2021, Porcellini et al. [39] reported the outcomes of 6 patients who underwent RTSA using custom-made glenoid components (ProMade, LimaCorporate) to treat severe glenoid defects. They noticed improvements in all clinical parameters at an average follow-up time of 31.67 months: the Constant-Murley score (CMS) increased by 9.83±5.60 points, and the ASES score increased by 30.57±10.77 points. They also reported an increase in the range of motion. Radiographic evaluations showed that two patients had a radiolucent line <2 mm; however, that did not change the outcome. Complications were encountered in two patients: one had postoperative pain that was gradually improving, and the other had a nontraumatic dislocation, possibly due to weakness in the anterior portion of the deltoid, which was treated with physical therapy and athletic taping.
A retrospective study by Ortmaier et al. [41] in 2022 evaluated 10 shoulders with severe glenoid bone loss undergoing revision RTSA using custom-made glenoid implants. Two types of custom glenoid implants were used: the Materialise (Glenius, Materialise NV) glenoid design was used for eight shoulders, and the Lima (ProMade, LimaCorporate) glenoid design was used for the remaining 2. At a mean follow-up of 23.1 months, clinical examination revealed statistically significant increases in CMS, University of California-Los Angeles (UCLA) score, and SSV. Their comparison of the intended and actual screw lengths revealed a mean accuracy of 95.4% in radiological assessment. In the posterior, superior, and medial directions, there were average differences of 2.9 mm, 0.9 mm, and 0.5 mm, respectively, between the anticipated implant location and the actual position. No loosening or fracture was noted. These radiological and clinical findings are consistent with the previously mentioned literature.
Subsection 3: Contraindications and Disadvantages
As with any new technology or surgical technique, the use of PSI is not flawless. First, patient-specific glenoid components should be reserved as a last resort for patients with severe glenoid bone loss that is deemed irreparable by other means. No contraindications specific to the use of PSI could be found in the literature, but the typical contraindications to RTSA remain applicable, such as severe deltoid impairment, infection, and neuropathic joints [49,50]. The disadvantages of PSI are still under study. As described earlier, the bulkiness of the component and its associated instruments can impair proper placement [43]. The precision of the implants, as well as the possibility of further bone erosion, means that surgery must be performed as soon as possible after the CT scan is obtained [43]. Restoration of the anatomical joint line automatically increases the distance from the center of rotation to the bone–metal interface, which increases the shear forces and could lead to the increased loosening rate sometimes noted during long-term follow up [41]. In the setting of revision arthroplasty, the previous components cause artifactual disturbances on the CT scan, which can hinder proper modeling of a custom glenoid baseplate and require a two-stage surgery [39,43]. In addition, financial considerations pose limitations on the use of modern PSIs. Compared with a conventional Comprehensive Reverse baseplate, which costs $3,700 USD, the VRS baseplate has a list price of $14,940 USD [38]. Finally, considering the novelty of this technique, few studies have evaluated the clinical and radiological outcomes, especially in the long term. Similarly, clear guidelines about use of these implants are unavailable, and the defect severity that necessitates custom implants remains a matter of controversy, with most surgeons deciding for themselves based on their personal and professional experience.
RECOMMENDATIONS
There is a paucity of research about the indications and outcomes of PSI in the setting of glenoid bone loss. As stated earlier, most management plans and decisions about this technology stem from personal and clinical experiences, so sharing our insights about the use of PSI could help other surgeons decide when and how to apply it. As such, we offer the following recommendations. (1) Meticulous care should be taken when choosing the right patients for PSI because the technology is relatively novel, and long-term outcomes remain equivocal. (2) The choice of technique to overcome glenoid bone loss must consider the needs of individual patients. Each of the relevant methods has its own strengths and limitations, and any surgical decision, be it excessive reaming, the use of an alternate centerline, bone grafting, or PSI, must be patient-centered and discussed with them. (3) Further studies need to be conducted to determine the short- and long-term outcomes of using PSIs in patients with severe glenoid bone loss. Prospective studies making detailed comparisons with the other relevant modalities could help establish the role of PSI in current treatment guidelines and strategies. In addition, reporting both positive and negative outcomes could improve operating techniques and decrease postoperative complications. (4) Studies about the cost effectiveness of PSIs are necessary to help clarify and define the financial implications of using this tool, as well as to determine the demographic that might benefit from it most. (5) Further biomechanical studies should be conducted to improve the implant design and structure and to determine the best material for optimal outcomes.
CONCLUSIONS
RTSA has become a popular treatment option for several shoulder pathologies. As with any surgery, an increase in prevalence often comes with increased number and severity of complications. Glenoid bone loss has become an increasingly difficult pathology to treat, especially in the setting of an aging population and an increase in the number of revision surgeries. Nevertheless, several techniques have been developed, ranging from excessive reaming to the newly described PSIs (custom-made glenoid components). Because PSIs are relatively new, there is a scarcity of literature on the topic, and most of it is extrapolated from hip-inspired custom implants. The studies available have shown promising results, with good patient satisfaction, improved clinical and radiographic scores, and a decrease in pain scores. However, the complication rates vary among studies and could be affected by surgeon experience and the type of implant used. Further studies are required to develop a standardized classification system, determine what pathologies require custom-made implants, evaluate the long-term outcomes of use, enhance operative techniques, evaluate their biomechanical properties, and assess the cost-effectiveness of their use.
Notes
Author contributions
Conceptualization: ERH, MYF. Supervision: JAA. Writing - original draft: ERH. Writing - review & editing: ERH, MYF, JAA.
Conflict of interest
None.
Funding
None.
Data availability
None.
Acknowledgments
None.