Influence of lateralized versus medialized reverse shoulder arthroplasty design on external and internal rotation: a systematic review and meta-analysis
Article information
Abstract
Background
Restoration of external (ER) and internal rotation (IR) after Grammont-style reverse shoulder arthroplasty (RSA) is often unreliable. The purpose of this systematic review was to evaluate the influence of RSA medio-lateral offset and subscapularis repair on axial rotation after RSA.
Methods
We conducted a systematic review of studies evaluating axial rotation (ER, IR, or both) after RSA with a defined implant design. Medio-lateral implant classification was adopted from Werthel et al. Meta-analysis was conducted using a random-effects model.
Results
Thirty-two studies reporting 2,233 RSAs were included (mean patient age, 72.5 years; follow-up, 43 months; 64% female). The subscapularis was repaired in 91% (n=2,032) of shoulders and did not differ based on global implant lateralization (91% for both, P=0.602). On meta-analysis, globally lateralized implants achieved greater postoperative ER (40° [36°–44°] vs. 27° [22°–32°], P<0.001) and postoperative improvement in ER (20° [15°–26°] vs. 10° [5°–15°], P<0.001). Lateralized implants with subscapularis repair or medialized implants without subscapularis repair had significantly greater postoperative ER and postoperative improvement in ER compared to globally medialized implants with subscapularis repair (P<0.001 for both). Mean postoperative IR was reported in 56% (n=18) of studies and achieved the minimum necessary IR in 51% of lateralized (n=325, 5 cohorts) versus 36% (n=177, 5 cohorts) of medialized implants.
Conclusions
Lateralized RSA produces superior axial rotation compared to medialized RSA. Lateralized RSA with subscapularis repair and medialized RSA without subscapularis repair provide greater axial rotation compared to medialized RSA with subscapularis repair.
Level of evidence
2A.
INTRODUCTION
Since its first description by Grammont in 1985 [1,2], the design of reverse shoulder arthroplasty (RSA) has evolved considerably. While effective at alleviating pain and improving overhead range of motion (ROM), initial reports showed poor restoration of satisfactory external and internal rotation (ER and IR, respectively) [3,4]. Consequently, these reports led many to believe that RSA leads to poor active ER and poor active and passive IR. Nevertheless, it appears that, despite these reported limitations in measured axial ROM, patients can manage toileting (which requires active IR) after unilateral and even after bilateral RSA [5-7].
It has been hypothesized that poor postoperative ER occurs with a lack of tensioning of the posterior rotator cuff secondary to medialization of the greater tuberosity. Similarly, it is believed that loss of passive IR is due to mechanical impingement between the humeral implant and the scapular neck. These hypotheses informed the evolution of the modern lateralized RSA, which is believed to provide tension to the posterior rotator cuff and posterior deltoid to restore active ER and increase impingement-free ROM, improving active and passive IR. However, clinical studies demonstrating a superior axial ROM for lateralized versus Grammont-style RSA are rare because they require surgeons to implant prostheses of varying designs. Furthermore, successful repair of the subscapularis is believed by many surgeons to influence postoperative ROM, although clinical evidence is variable [8-10].
The purpose of this systematic review was to evaluate the influence of RSA medio-lateral offset on axial rotation after RSA. Secondarily, we sought to assess the influence of the interaction between medio-lateral implant offset and subscapularis management (repair or spared versus no repair) on axial rotation. We hypothesized that lateralized RSA would be associated with superior axial rotation.
METHODS
This systematic review was performed in accordance with the guidelines for Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [11].
Eligibility Criteria
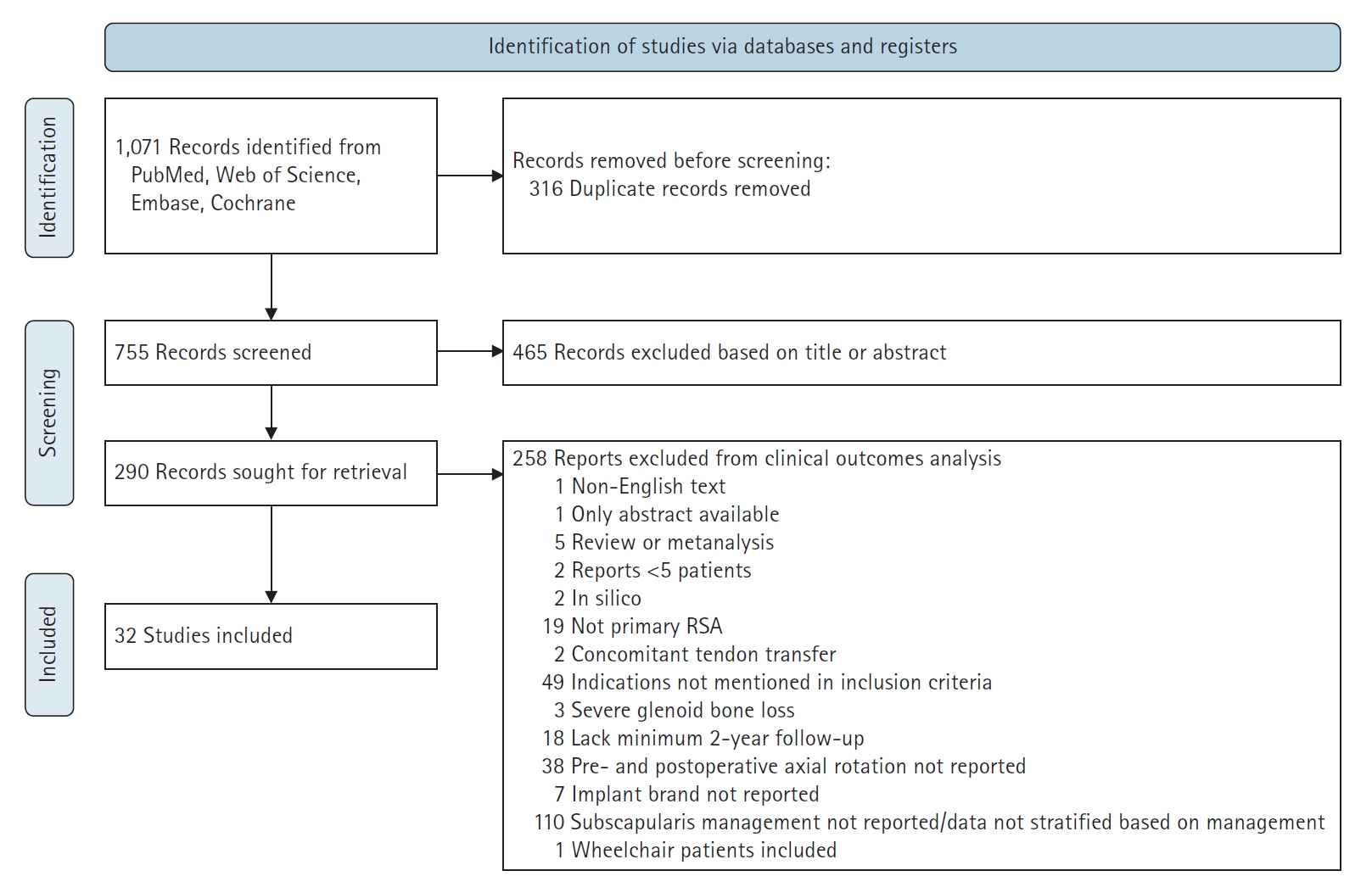
We included original studies reporting on axial rotation after RSA for cuff tear arthropathy, irreparable rotator cuff tear, primary osteoarthritis, or osteoarthritis with rotator cuff deficiency. Studies were excluded if they were a duplicate, written in a language other than English, a review or meta-analysis, a case report or series reporting on fewer than five patients, a commentary or editorial, part of the gray or unpublished studies, an in silico (computer simulation) or in vitro (purely biomechanical or anatomical) study, not assessing primary RSA (e.g., revision RSA), lacking at least 2 years (≥21 months) of follow-up, considering concomitant tendon transfer, including RSA for severe glenoid bone loss, or using a custom glenoid. Studies including patients undergoing RSA for indications other than those specified in the inclusion criteria (post-traumatic arthritis, rheumatoid arthritis, post-infectious arthritis, revision arthroplasty, avascular necrosis, humerus malunions) were also excluded. Studies that did not report postoperative axial rotation, implant manufacturer and model, or subscapularis management were excluded. Finally, study populations of exclusively wheelchair-dependent patients were excluded. The detailed study inclusion and exclusion process is shown using a PRISMA diagram in Fig. 1. Risk of bias was assessed by a single investigator (CG) through use of the Methodological Index for Non-Randomized Studies (MINORS) criteria [12]. Individual studies and MINORS scores are indicated in Supplementary Table 1 [13-44].
Search Strategy
An experienced medical librarian (DAD) implemented a comprehensive literature search to identify English language papers on the influence of the design of RSA on axial rotation published between January 2010 and December 2019. To locate relevant studies, we queried PubMed, Embase, Scopus, the Web of Science, and the Cochrane Database of Systematic Reviews. Search terms included a combination of keywords and subject headings including “primary RSA,” “reverse shoulder arthroplasty,” “prosthetic,” “reverse shoulder replacement,” “cuff tear arthropathy,” “design,” “axial rotation,” “range of motion,” and “prosthesis” (see the Supplementary Material 1 or database-specific search strategies). In addition to limiting the results to English, they were limited to adults and the following publication types: randomized controlled trials, controlled clinical trials, clinical trials, evaluation studies, case–control studies, cohort studies, longitudinal studies, prospective studies, retrospective studies, follow-up studies, comparative studies, systematic reviews, and meta-analyses (Supplementary Material 1 for database-specific search strategies). The searches resulted in 1,071 total results. After a three-step de-duplication process in Endnote, 755 original studies remained. Exclusion criteria were applied to title and abstract screening by three authors (RJC, CG, and DAF), and, when questionable, we erred on the side of inclusion. Subsequently, full texts of the remaining articles were reviewed by the same authors. Discrepancies and uncertainties were resolved by involving the lead author (KAH); when agreement among all team members could not be reached, expert opinion was provided by a panel of senior shoulder and elbow surgeons (JJK, BSS, and JDW).
Data Extraction
Data extraction was completed by three authors (RJC, CG, and DAF) using a standardized data-collection form. Any disagreements were discussed with the lead author (KAH), and a panel of senior shoulder and elbow surgeons (JDW, JJK, TWW, BSS) was consulted to arbitrate any unresolved issues. Data extracted from the articles included in the final review included level of evidence, country of the corresponding author, whether the study was retrospective or prospective, number of shoulders, number of total patients, mean length of follow-up, average age, sex, ROM in ER, medialization vs. lateralization classification of prostheses [45], and the complications of interest. Although highly relevant, we anticipated substantial heterogeneity in the reporting of IR as described previously; thus, it was considered a secondary outcome of this study [46].
Data Analysis and Synthesis
Study characteristics were summarized descriptively. Weighted means, based on the number of RSAs in each study, were calculated for study demographics and characteristics of interest. Implant lateralization was based upon prior work by Werthel et al. [45], who reviewed digitized templates of 28 configurations with 22 different implants and assessed glenoid, humeral, and global lateralization normalized to the Delta III prosthesis (DePuy Synthes). In addition to classifying each implant into one of two glenoid categories (medialized or lateralized) and one of three humeral categories (medialized, minimally lateralized, and lateralized humerus), implants were separated into categories of 5-mm increments for global offset (medialized, minimally lateralized, lateralized, highly lateralized, and very highly lateralized). To enable meaningful comparison, the global implant lateralization of implants from included series was determined according to the method of Werthel et al. [45] and classified as medialized (medialized and minimally lateralized) or lateralized (lateralized, highly lateralized, and very highly lateralized). Studies that reported outcomes of multiple treatment strategies that we defined to be of interest for comparison a priori (i.e., prosthesis lateralization, subscapularis repair) were recorded as separate cohorts to facilitate meta-analysis. Thus, outcomes analysis was performed on 38 patient cohorts reported in 32 studies.
The weighted mean preoperative and postoperative ER values were calculated. Due to substantial heterogeneity in IR reporting, we summarized preoperative and postoperative IR values descriptively and assessed whether they achieved or exceeded the minimum necessary internal rotation (MNIR) needed to perform activities of daily living [46]. The MNIR has been previously reported to be 79° or the L3 vertebral level [7,47]. Meta-analysis was performed to compare the postoperative ER and pre- to postoperative improvement in ER based on global implant design (lateralized vs. medialized) and further stratified based on management of the subscapularis (repair with any method versus no repair). We anticipated that the design of the included studies and methodology involved in data collection would result in substantial heterogeneity; thus, we elected to use a random-effects model a priori [48]. The I2 statistic was used to assess the heterogeneity of results. The true effect size in 95% of the population (95% prediction interval) was calculated using the variance of true effects (T2) and the standard deviation of true effects (T). Meta-analysis was performed using the metafor R package [49]. All statistical analyses were performed using R (version 4.2.0; R Core Team) with an α value of 0.05.
RESULTS
Thirty-two studies reporting on 2,233 shoulders were included. The mean MINORS score was 13.4/16 points for non-comparative studies and 20.3/24 points for comparative studies. Included patients had a weighted mean age of 72.5 years (range, 66–81 years), a mean follow-up of 43 months (range, 24–97 months), a minimum follow-up of 30 months (range, 24–62 months), and 64% of patients were female. The subscapularis was repaired in 91% (n=2,032) shoulders; this did not differ based on use of a globally lateralized versus medialized implant (91% [884/967] vs. 91% [1,148/1,266], P=0.602). Mean preoperative ER was reported by 27 of the 32 included studies and had a weighted mean of 21.6° (range, 0°–45°). Mean postoperative ER was reported by all included studies and had a weighted mean of 31.2° (range, 9.9°–54°).
ER Based on Global Lateralization
The meta-analysis of postoperative ER based on global implant lateralization included 22 studies (11 lateralized cohorts, 17 medialized cohorts) reporting on 1,467 RSAs (598 lateralized, 869 medialized) (Fig. 2). According to the analysis, postoperative ER was significantly greater in cohorts with globally lateralized versus medialized implants (40° [36°–44°] vs. 27° [22°¬–32°], P<0.001). Separately, the meta-analysis of pre- to postoperative improvement in ER based on a globally lateralized versus medialized implant included 17 studies (8 lateralized cohorts, 11 medialized cohorts) reporting on 1,210 RSAs (552 lateralized, 658 medialized) (Fig. 3) and determined that pre- to postoperative improvement in ER was significantly greater in cohorts with globally lateralized versus medialized implants (20° [15°–26°] vs. 10° [5°–15°], P<0.001).
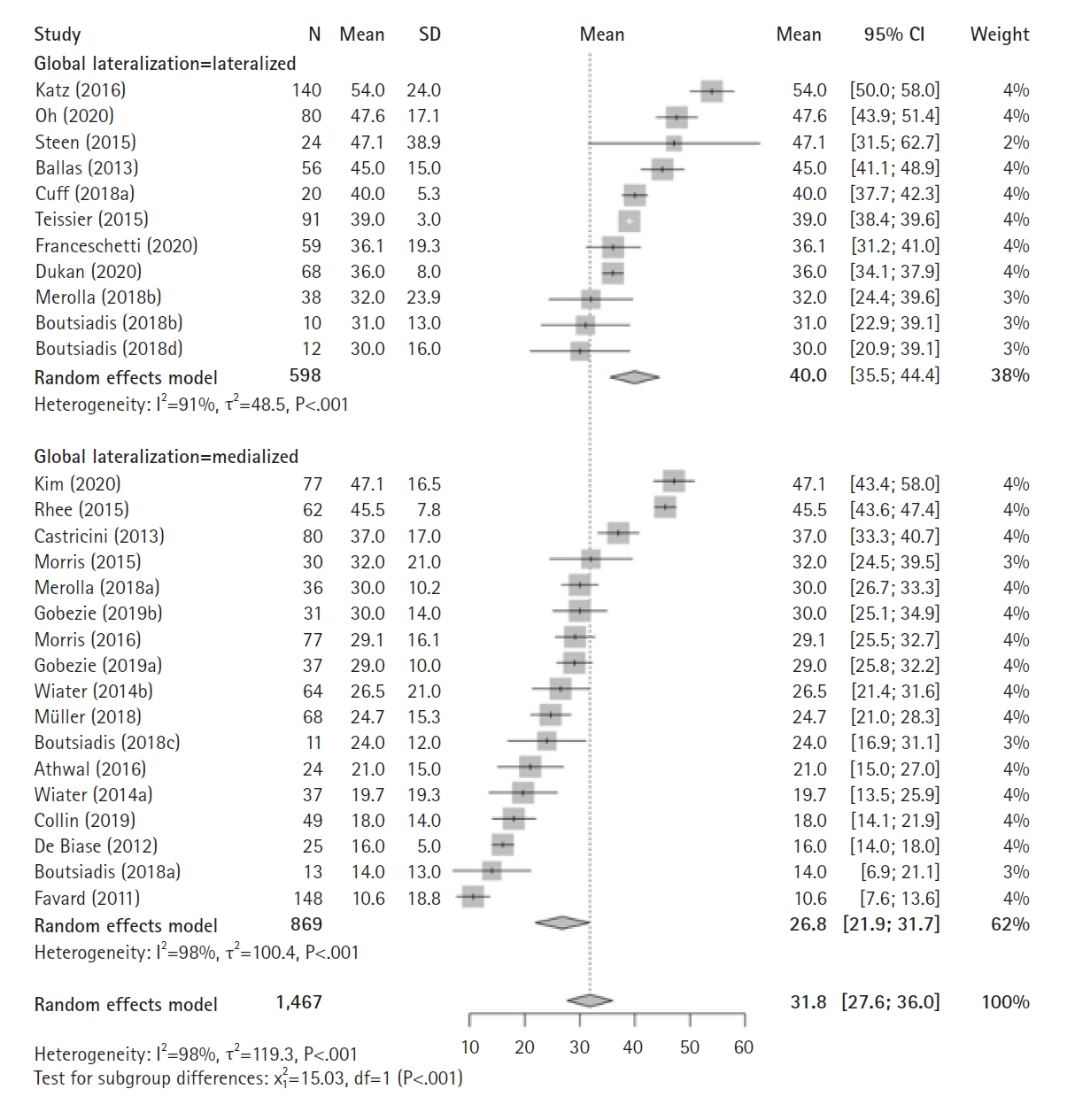
Forest plots of the mean postoperative external rotation after reverse shoulder arthroplasty using a globally lateralized versus medialized prosthesis. SD: standard deviation, MD: mean difference, CI: confidence interval.
ER Based on Global Lateralization and Subscapularis Management
The meta-analysis of postoperative ER based on global implant lateralization and subscapularis repair included 22 studies (11 lateralized with subscapularis repair, 15 medialized with subscapularis repair, 2 medialized without subscapularis repair) reporting on 1,467 RSAs (598 lateralized with repair, 759 medialized with repair, 110 medialized without repair) (Fig. 4). This analysis determined that postoperative ER was significantly greater in cohorts with lateralized implants with subscapularis repair (40° [36°–44°]) or medialized implants without subscapularis repair (36° [31°–40°]) than those with globally medialized implants with subscapularis repair (26° [21°–31°], P<0.001). The meta-analysis of pre- to postoperative improvement in ER based on global implant lateralization and subscapularis repair included 17 studies (8 lateralized with subscapularis repair, 9 medialized with subscapularis repair, 2 medialized without subscapularis repair) reporting on 1,210 RSAs (552 lateralized with repair, 548 medialized with repair, 110 medialized without repair) (Fig. 5) and found that pre- to postoperative improvement in ER was significantly greater in cohorts with lateralized implants with subscapularis repair (20° [15°–26°]) or medialized implants without subscapularis repair (21° [17°–25°]) compared to those with globally medialized implants with subscapularis repair (8° [3°–13°], P<0.001).

Forest plots of the mean postoperative external rotation after reverse shoulder arthroplasty using a globally lateralized versus medialized prosthesis with versus without subscapularis repair. SD: standard deviation, MD: mean difference, CI: confidence interval.
Internal Rotation
The mean postoperative active IR was reported by 56% (n=18) of studies (9 lateralized and 12 medialized cohorts) (Table 1) [13,15-17,24,27-31,33-36,38,40,43]. The mean postoperative IR achieved the MNIR for 51% of patients with lateralized implants (n=325, 5 cohorts) versus 36% of patients with medialized implants (n=177, 5 cohorts) (P<0.001). The IR reporting methods included degrees in angle (n=4), vertebral level (n=7), and IR sub-score from the Constant score (n=6).
DISCUSSION
Lateralized RSA is hypothesized to better restore axial rotation compared to the original Grammont-style prosthesis; however, appropriately powered comparative cohort studies controlling for surgeon implant choice are impractical. The present systematic review and meta-analysis of 2,233 RSAs reported in 32 articles found that studies performing globally lateralized RSA were associated with greater preoperative to postoperative improvement in active ER and greater postoperative ER using a meta-analysis comparison. Furthermore, lateralized RSA with subscapularis repair or medialized RSA without subscapularis repair provided superior ER compared to medialized RSA with subscapularis repair. While meta-analysis of IR was not feasible, we found that a greater proportion of patients exceeded the MNIR in studies enrolling patients who underwent lateralized RSA.
Historical studies of the Grammont prosthesis reported no improvements in ER after revision TSA [4,50]. Poor restoration of ER with the Grammont prosthesis is often attributed to the medialized glenoid–medialized humerus design, which is hypothesized to inadequately tension any existing posterior rotator cuff and posterior deltoid compared to contemporary lateralized RSA designs. Although tension of the posterior cuff may be maintained through distalization of the tendon insertions with the Grammont design, their line of action becomes more oblique, effectively reducing the moment arm. Our results demonstrating superior active ER with lateralized RSA support these hypotheses and corroborate a recent meta-analysis of 440 lateralized and 425 Grammont-style RSAs, which found greater ER with use of a lateralized implant [51].
Prior meta-analyses have been conducted to compare axial ROM after lateralized versus medialized RSA [3,5,51]; however, their inclusion and exclusion criteria vary. Cho et al. [5] included five studies that compared lateralized (n=346) versus medialized (n=217) RSA and found no difference in postoperative ER (standardized mean difference, 0.21 [−0.14 to 0.56]; P=0.238); however, pre- to postoperative improvement in ER favored lateralized RSA in their analysis of two studies (standardized mean difference, 0.71 [0.36–1.07]; P<0.001). Although Cho et al. [5] similarly classified implant lateralization using the classification proposed by Werthel et al., [45], the authors only included comparative studies, limiting study inclusion. Furthermore, the authors did not specifically exclude studies with patients with preoperative diagnoses associated with poorer prognoses (i.e., post-traumatic, post-infectious, inflammatory arthritis, revision arthroplasty), severe bone loss, concomitant tendon transfer, or less than 2 years of follow-up. Berton et al. [3] performed a meta-analysis and found greater pre- to postoperative improvement in ER for lateralized (n=802, 5 studies) versus medialized (n=220, 7 studies) RSA (mean difference, 20.4° [17.6°–23.1°] vs. 8.3° [5.9°–10.7°]; P<0.01). They also found that lateralized versus medialized RSA had lower rates of scapular notching (6.6% vs. 47.7%, P<0.01) and postoperative infection (1% vs. 7.7%, P=0.01). Berton et al. [3] used stricter inclusion and exclusion criteria in their study compared to Cho et al., [5] and their work was more similar to the present study, as they included studies reporting on patients with indications limited to cuff tear arthropathy, irreparable cuff tear, or cuff tear associated with osteoarthritis. Although Berton et al. [3] also excluded studies including patients undergoing revision RSA or an indication of rheumatoid arthritis, acute fracture, post-traumatic fracture sequelae, tumor, or active infection, the authors only required 1 year of follow-up. Since ROM is known to continue to improve up to 2 years postoperatively [52], the findings of Berton et al. [3] may not accurately represent the full improvement that occurs with lateralized implants. Despite variations in inclusion and exclusion criteria, most prior meta-analyses agree that lateralized RSA provides superior ER compared to medialized RSA, with no consistent differences in clinical outcome scores across studies.
Though a few prior meta-analyses have attempted to assess the effects of both lateralization and subscapularis management, prior studies have not been able to demonstrate a difference in ER. Kim et al. [53] performed a meta-analysis of comparative studies and compared outcomes after lateralized RSA with glenoid-sided (n=257, 7 studies) versus humeral-sided (n=95, 4 studies) lateralization per Werthel et al. [45] and found no difference in ER (mean difference, 3.1°; 95% CI, −8.9° to 15.2°), forward elevation (mean difference, 1.0°; 95% CI, −13.4° to 15.5°), or ASES and Constant scores. However, a subgroup analysis of six studies involving concomitant subscapularis repair demonstrated that humeral-sided lateralization had more favorable forward elevation (mean difference, 26.1°; 95% CI, 8.5°–43.7°) but similar ER values (10.1°; 95% CI, −9.1° to 29.2°) and ASES and Constant scores compared to glenoid-sided lateralization. Corona et al. [54] performed a meta-analysis of two comparative cohort studies comparing lateralized RSA with (n=378) versus without (n=289) subscapularis repair and found no difference in postoperative ER (mean difference, −1.2°; 95% CI, −3.9° to 1.5°; P=0.39) or postoperative forward elevation (mean difference, 3.9°; 95% CI, −0.4° to 8.1°; P=0.07) but a greater postoperative IR when scored per Flurin et al. [6] (mean difference, 0.68; 95% CI, 0.46–0.89; P<0.001). In contrast, we demonstrated that use of a lateralized implant with subscapularis repair or medialized implant without subscapularis repair was associated with significantly greater postoperative ER (Fig. 4) and pre- to postoperative improvement in ER (Fig. 5) compared to using a globally medialized implant with subscapularis repair (P<0.001 for both). We believe these results suggest that tensioning of the posterior rotator cuff and deltoid with lateralization offsets the force couple of the repaired subscapularis, providing a similar ER compared to that achieved with the use of a medialized implant without subscapularis repair. Notably, the combined use of a medialized implant with subscapularis repair may provide inadequate tensioning of the external rotators to overcome the added force couple of the repaired subscapularis. However, use of a medialized implant without subscapularis repair may be unfavorable; Matthewson et al. [55] performed a meta-analysis of 1,306 patients from seven studies and recorded a greater dislocation rate when the subscapularis was not repaired (24/583 [4.1%] vs. 5/723 [0.7%], odds ratio [OR]=0.24, P=0.04). Although a trend toward a lower risk of dislocation with subscapularis repair was found when pooling studies with a lateralized RSA only, the difference was not significant (OR=0.29, P=0.07). A single study using a medialized implant found a lower dislocation rate with subscapularis repair (0.6% vs. 11%, OR=0.05, P=0.004). Unfortunately, no studies enrolling lateralized RSA patients without subscapularis repair were included in the present study. In theory, this technique may provide the greatest benefit to ER after RSA, although it has potential risk of poor active IR.
Although we were unable to perform a meta-analysis to compare IR due to heterogeneity in reporting by included studies, we found that a greater proportion of patients undergoing lateralized versus medialized RSA exceeded the MNIR (51% vs. 36%, P<0.001). The proportion of patients undergoing subscapularis repair was similar between lateralized and medialized RSA cohorts among the patients included in our study. Notably, only the study by Corona et al. [54] of all aforementioned systematic reviews attempted to meta-analyze IR and ultimately found more favorable IR (per the scale proposed by Flurin et al. [6]) with subscapularis repair versus without when a lateralized RSA was used; however, only two studies and 378 patients were included in this assessment. Together, these findings suggest that use of lateralized versus medialized RSA can provide superior ER without IR detriment, especially when the subscapularis is repaired.
This systematic review and meta-analysis is not without its limitations. Foremost, the inclusion of many retrospective studies means there are possible individual and compounded reporting biases. Additionally, the quality of our review is dictated by the quality of individual studies included. None of the studies included in the meta-analysis of ER based on both implant design and subscapularis management included patients receiving lateralized implant without subscapularis repair; biomechanically, this is believed to portend the greatest ER without sacrificing implant stability. Future studies are needed to evaluate whether lateralized RSA without subscapularis repair provides optimal ER while maintaining a low rate of postoperative instability. Although at least 2 years of clinical follow-up was required, included studies had varying follow-up periods. Furthermore, publication bias is a potential limitation (Fig. 6). The assessment of ER was not uniform: 11 studies assessed ER with the shoulder adducted at the side, one study assessed ER at 90° of abduction, and the remainder of the studies did not specify the position of the arm. We were also unable to meaningfully analyze IR due to substantial heterogeneity in reporting; this is a known limitation that has previously been reported and remains controversial [46]. Moreover, in our attempt to capture all relevant articles, we queried commonly used databases with broad search terms; however, despite these efforts, relevant articles may have been missed. Despite these limitations, this was a large-scale review and meta-analysis that contributes to the current literature and knowledge regarding the influence of prosthesis lateralization on axial rotation after RSA.
CONCLUSIONS
Lateralized RSA produces superior axial rotation compared to medialized designs. Lateralized RSA with subscapularis repair and medialized RSA without subscapularis repair provide greater postoperative ER and pre- to postoperative improvement in ER compared to medialized RSA with subscapularis repair.
Notes
Author contributions
Conceptualization: KAH, JJK, TWW, DAD, BSS, JDW. Data curation: KAH, RJC, CG, DAF, JJK, TWW, DAD, BSS, JDW. Formal analysis: KAH, JJK, TWW, BSS, JDW. Investigation: KAH, RJC, JJK, TWW, BSS, JDW. Methodology: KAH, JJK, TWW, BSS, JDW. Project administration: KAH, JJK, TWW, BSS, JDW. Resources: JJK, TWW, DAD, BSS, JDW. Supervision: KAH, RJC, JJK, TWW, BSS, JDW. Validation: KAH, JJK, TWW, BSS, JDW. Visualization: KAH, JJK, TWW, BSS, JDW. Writing – original draft: KAH, JJK. Writing – review & editing: KAH, CG, DAF, JJK, TWW, BSS, JDW.
Conflict of interest
KAH has a consultancy agreement with LinkBio Corp. JJK is a paid consultant for Exactech, Inc. and LinkBio Corp. Dr. Wright is a paid consultant and receives royalties from Exactech, Inc. BSS is a paid consultant and receives royalties from Exactech, Inc.; Responsive Arthroscopy; and Innomed. The other authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Funding
None.
Data availability
Contact the corresponding author for data availability.
Acknowledgments
None.
SUPPLEMENTARY MATERIALS
Supplementary materials can be found via https://doi.org/10.5397/cise.2023.00577.
List of included articles
12.9.20 PubMed (564)