Discovery Elbow System arthroplasty polyethylene bearing exchange: outcomes and experience
Article information
Abstract
Background
The Discovery Elbow System (DES) utilizes a polyethylene bearing within the ulnar component. An exchange bearing requires preoperative freezing and implantation within 2 minutes of freezer removal to allow insertion. We report our outcomes and experience using this technique.
Methods
This was an analysis of a two-surgeon consecutive series of DES bearing exchange. Inclusion criteria included patients in which exchange was attempted with a minimum 1-year follow-up. Clinical and radiographic review was performed 1, 2, 3, 5, 8 and 10 years postoperative. Outcome measures included range of movement, Oxford Elbow Score (OES), Mayo Elbow Performance Score (MEPS), complications and requirement for revision surgery.
Results
Eleven DESs in 10 patients were included. Indications were bearing wear encountered during humeral component revision (n=5); bearing failure (n=4); and infection treated with debridement, antibiotics and implant retention (DAIR; n=2). Bearing exchange was conducted on the first attempt in 10 cases. One case required a second attempt. One patient developed infection postoperatively managed with two-stage revision. Mean follow-up of the bearing exchange DES was 3 years. No further surgery was required, with no infection recurrence in DAIR cases. Mean elbow flexion-extension and pronosupination arcs were 107° (±22°) and 140° (±26°). Mean OES was 36/48 (±12) and MEPS was 83/100 (±19).
Conclusions
Our results support the use of DES bearing exchange in cases of bearing wear with well-fixed stems or acute infection. This series provides surgeons managing DES arthroplasty with management principles, successful and reproducible surgical techniques and expected clinical outcomes in performing DES polyethylene bearing exchange.
Level of evidence
IV.
INTRODUCTION
The Discovery Elbow System (DES; LimaCorporate) is a semi-constrained total elbow arthroplasty that utilizes a spherical hinge bearing (Fig. 1) [1]. Theorized to minimize polyethylene wear [2], the bearing comprises two cobalt chromium molybdenum hemispheres locked into the distal humeral component with medial and lateral Ti6Al4V screws. A congruent ArCom polyethylene bearing surface is captured within the proximal ulnar component with a locking pin [2].
The bearing design is intended to “allow for simple polyethylene exchange” [2] when clinically indicated. However, prior to insertion the polyethylene bearing requires freezing between –25 °C and –10 °C for a minimum of three hours to ensure sufficient constriction to insert into the ulnar component ring. Furthermore, upon removing the polyethylene bearing from the freezer, the bearing will begin to expand immediately and reach full expansion within 2 minutes of removal [3].
The time-critical nature of new bearing insertion may result in surgeon anxiety as 2 minutes is a relatively short period in which to retrieve a bearing from a freezer, perform a necessary “stop moment” to ensure the correct implant has been selected, transfer the polyethylene bearing to the operating surgeon and perform implantation. While removal of a component with loosening and removal of both humeral and ulnar components is necessary in chronic infection, a polyethylene bearing exchange may be indicated in a DES with bearing failure and well-fixed humeral and ulnar components. An exchange may also be necessary for a worn bearing encountered during revision of an aseptically loose humeral component with a well-fixed ulnar component that avoids the need for ulnar component revision. Acute infection is another indication for exchange, but there is a lack of published scientific evidence reporting surgeon experience or clinical outcomes in performing DES polyethylene bearing exchange. Given the paucity of evidence related to DES polyethylene bearing exchange, we aimed to report our experience, including patient preoperative evaluation for suitability, surgical technique and clinical outcomes.
METHODS
Institutional Review Board of University Hospitals of Derby and Burton NHS Foundation Trust's approval for this studyf was obtained (No. UHDBS355). Patient consent was obtained for all procedures. Inclusion criteria for this study included all patients for whom a DES polyethylene bearing exchange was attempted with a subsequent minimum 1-year follow-up. All patients underwent bearing exchange for one of the three indications: bearing failure, worn bearing encountered during revision of an aseptically loose humeral component or acute infection. Contraindications to attempting DES polyethylene bearing exchange included the identification of a loose ulnar component, ulnar component damage that compromised insertion of a polyethylene bearing or its locking pin, or chronic infection of the arthroplasty.
Indications for DES Polyethylene Bearing Exchange
DES polyethylene bearing exchange may be indicated in a DES with well-fixed humeral and ulnar components, but with bearing failure. An exchange may also be necessary for a worn bearing encountered during revision of an aseptically loose humeral component; the ulnar component must be well-fixed in these cases to avoid ulnar component revision. The presence of acute infection is a third indication for exchange.
Bearing failure
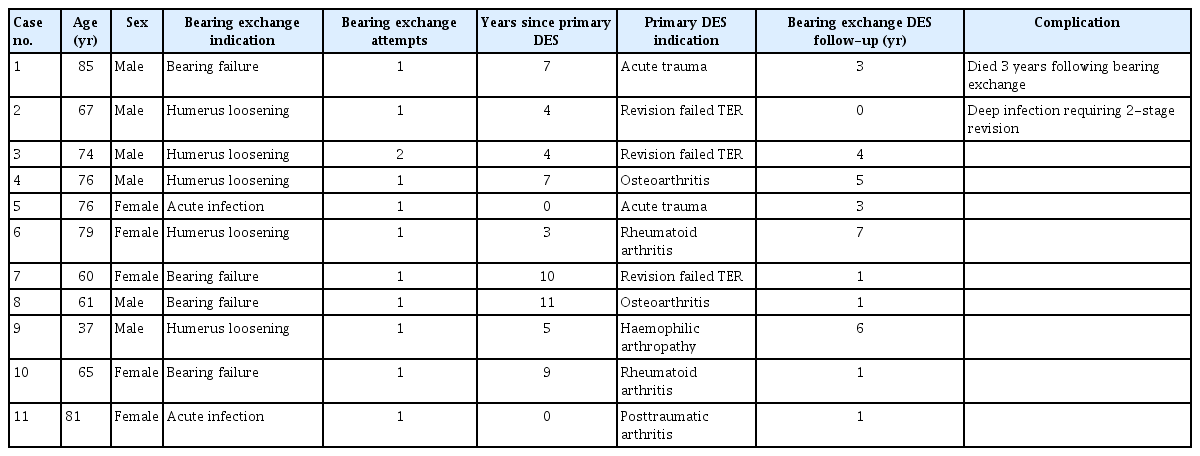
DES bearing failure may present as sudden mechanical failure of the arthroplasty as the polyethylene bearing locking pin disengages due to catastrophic wear of the adjacent polyethylene (Fig. 2). Locking pin disengagement can result in polyethylene bearing migration from the ulnar component. Patients report sudden loss of function that is typically accompanied by elbow crepitus and mechanical symptoms. This can occur without loosening of humeral and ulnar components.
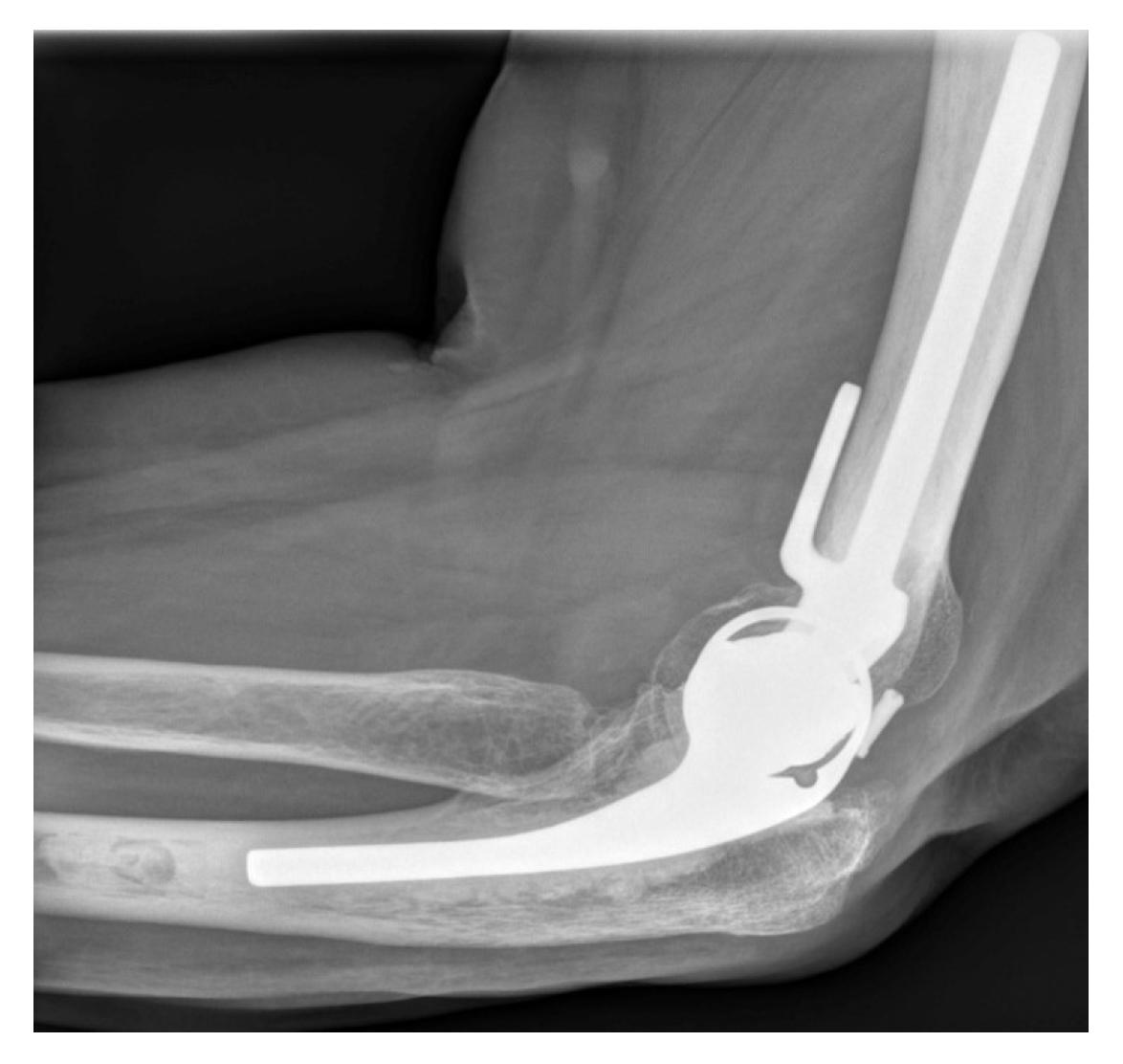
Lateral radiograph of a Discovery Elbow System prosthesis with a disengaged polyethylene bearing locking pin due to catastrophic wear of the adjacent polyethylene.
However, prior to catastrophic failure, bearing failure may present with a painful or painless effusion and associated functional deterioration. In our experience, patients do not report symptoms consistent with increased varus/valgus laxity. True-lateral radiographic assessment may reveal thinning of the polyethylene bearing (Fig. 3A), although this can be difficult to quantify. However, a difference in polyethylene thickness is evident radiographically when comparing a worn and recently-exchanged DES (Fig. 3B). Bearing failure may be considered a diagnosis of exclusion in a prosthesis of sufficient age when all other potential causes of symptoms, particularly aseptic loosening and infection, have been excluded. Well-fixed humeral and ulnar stems and exclusion of infection are imperative for polyethylene bearing exchange to be an effective treatment for bearing failure.

(A) Lateral radiograph of a Discovery Elbow System (DES) prosthesis with evident thinning of the polyethylene bearing and well-fixed humeral and ulnar components. Symptoms included painful effusion and functional deterioration. (B) Lateral radiograph of a DES prosthesis with polyethylene thickness restored following bearing exchange. Humeral and ulnar components were well-fixed during revision surgery and full symptom resolution was achieved.
In catastrophic bearing failure, the ulnar component ring that accommodates the polyethylene bearing and locking pin may be damaged. This may prevent subsequent stable insertion of replacements. An ulnar component revision would be required if this situation were encountered intraoperatively. Malpositioned ulnar or humeral components can contribute to premature bearing failure. If this condition is identified during a planned bearing exchange, we advise revision of the malpositioned component.
Worn bearing encountered during revision of an aseptically loose humeral component
Macroscopic wear of the polyethylene bearing may be encountered during revision of an aseptically loose humeral component; therefore, plans should be made preoperatively to facilitate a concomitant polyethylene bearing exchange. Polyethylene wear may have contributed to humeral component loosening via a histiocytic response to wear debris [4] , and allowing a worn bearing to remain in situ risks progression to bearing failure or subsequent aseptic loosening of the revised humeral or ulnar components. Preoperative evaluation should include exclusion of infection and careful assessment of ulnar component fixation, as revision would be recommended if the ulnar component were also loosening.
Acute infection
Evidence- and consensus-based guidelines of the British Elbow and Shoulder Society (BESS) [5] recommend debridement, antibiotics and implant retention (DAIR) in Yamaguchi type 1 cases (infection with stable implant) [6]. Infected elbow arthroplasty within three months of implantation and within a duration of symptoms of less than 3 weeks is the recommendation. BESS also recommends that DAIR include exchange of all elements of the prosthesis that can be removed without stem extraction and include all bushing and humeral spools whenever possible. Consequently, polyethylene bearing exchange is recommended in DES DAIR.
BESS guidelines clarify that DAIR is an appropriate strategy with “good” soft tissue cover. DAIR is appropriate for application against an infecting organism with the use of an antibiotic demonstrating effectiveness against the organism and biofilm production. Consequently, infections not fulfilling these criteria are unlikely to be adequately addressed with DAIR and two-stage revision may be indicated. The surgical strategy may be guided by prior joint aspiration and discussion with a microbiologist with expertise in periprosthetic infection.
Surgical Technique
Following preoperative confirmation of a suitable indication for polyethylene bearing exchange, obtaining records confirming the patients’ in situ DES implants is essential. Although the standard “Discovery ulna bearing revision kit” contains a suitable polyethylene bearing and locking pin for sizes 3, 4 and 5 Discovery ulnar components, the Discovery XS ulnar component (2.5×53 mm; 2.5×84 mm) requires the “Discovery XS ulna bearing revision kit.” An appropriate Discovery humeral condyle kit containing two condyles and two screws is also required.
Manufacturers recommend having two suitable “Discovery ulna bearing revision kits” available and located within a suitable freezer at the time of polyethylene bearing exchange. Freezer location should be considered to ensure minimal delay in retrieving and transferring a bearing to the operating surgeon. Bearing revision kits should be placed in a freezer for a minimum of three hours preoperatively. Patient consent should include discussion regarding the influence of intraoperative findings upon the subsequent procedure performed. Malpositioned components, unexpected ulnar component damage or loosening, frank infection or failure to insert a polyethylene bearing would necessitate revision of the ulnar component.
Our surgical technique in performing a primary DES implantation has been reported previously [7]. Pertinent variation in performing DES bearing exchange includes universal use of a midline triceps split approach as described by Gschwend et al. [8] in which the triceps insertion is elevated from the olecranon using a fine osteotome. Care should be taken to ensure that periosteal and osteal flakes are mobilized with the triceps tendon to facilitate some osseous on-growth following subsequent repair. This approach provides complete exposure to the arthroplasty and permits unrestricted access to the DES ulnar component. This is essential for application of both the DES bearing removal tool and ulnar pin inserter.
The joint capsule is frequently tarnished with metallosis, particularly in cases of bearing failure in which the DES cobalt chromium molybdenum condyles have undergone resultant abrasive wear (Fig. 4). Attempts should be made to excise all metallosis. The arthroplasty should be unlinked by removing the medial and lateral Ti6Al4V screws and condyles. The distal humerus and proximal ulna should be mobilized via soft tissue release, and the humeral and ulnar components should be carefully inspected. Contraindications for proceeding with bearing exchange, including frank infection, a loose ulnar component or a significantly damaged ulnar component, should be excluded. The ulnar component ring is often able to translate over a worn polyethylene bearing; this is an abnormal finding.
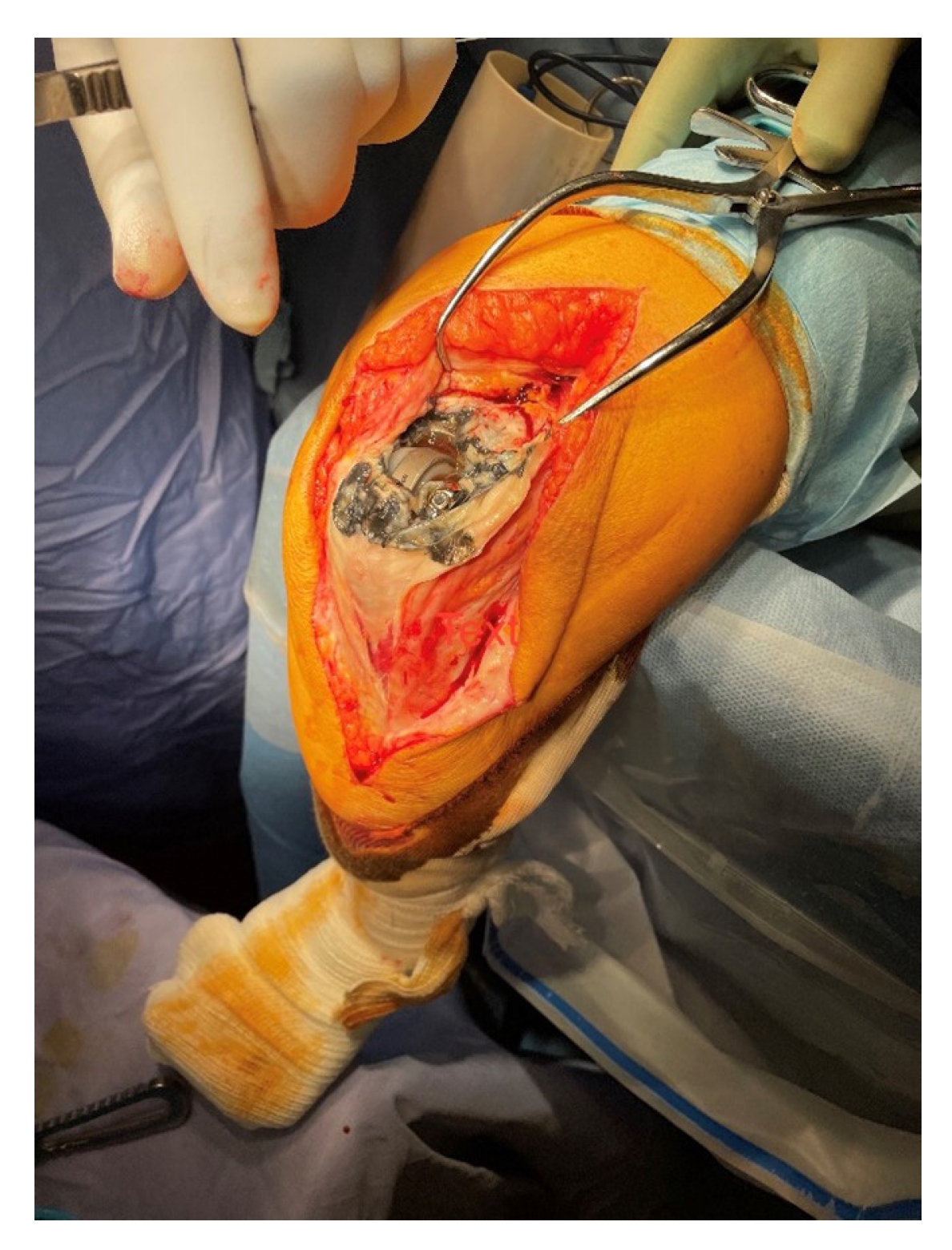
Joint capsule tarnished with metallosis during Discovery Elbow System bearing exchange for bearing failure.
Polyethylene bearing exchange is performed as per the manufacturer’s surgical technique [3] instructions with the first step being bearing removal. If the bearing exchange indication is acute infection, biopsy and radical debridement to remove any necrotic or obviously infected soft tissue and thorough lavage with at least 6 liters of saline is performed as per BESS guidelines [5]. If bearing exchange is being performed concurrently with a humeral component revision, the humeral component should then be addressed.
A new polyethylene bearing should be inserted within 2 minutes of freezer removal. One frozen suitable bearing revision kit should be retrieved and a “stop moment” performed to ensure the correct implant has been selected. The polyethylene bearing is transferred to the surgeon in a sterile manner and inserted appropriately. If the operating surgeon is unable to insert the bearing within 2 minutes, a second attempt is made using the remaining frozen suitable bearing revision kit. If both attempts are unsuccessful, an ulnar component revision is necessary. The ulnar pin inserter and a suitable humeral condyle kit are then used to link the arthroplasty. Range of motion should be assessed, and any impinging bone should be identified and removed. Thorough lavage with saline should be performed, and the triceps tendon should be repaired utilizing transosseous high-strength non-absorbable sutures as described by Gschwend et al. [8]. The tourniquet is deflated prior to closure to ensure adequate hemostasis.
Postoperative Care and Rehabilitation
Postoperative compressive bandaging is used for 48 hours and, in the absence of specific wound healing concerns, patients are permitted full range of movement upon expiration of regional anesthesia. Only gravity-assisted triceps function is allowed for 6 postoperative weeks with return to functional activities and progressive loading up to a maximum of 3 kg thereafter [7]. Patients requiring bearing exchange for bearing failure or a worn bearing encountered during revision of an aseptically loose humeral component are discharged from the hospital the following day with oral analgesia. Patients requiring bearing exchange during DAIR for acute infection receive broad-spectrum intravenous antibiotics as per local microbiology guidelines pending microbiology results from the intraoperative biopsy. Patients are examined by their operating surgeon 2 weeks postoperatively to ensure wound healing and determine the requirement for a night extension splint. Subsequent follow-up clinical assessment, radiograph and completion of patient-reported outcome measures (PROMs) are performed at 1 year postoperatively.
Outcomes
Clinical outcomes were obtained via prospective analysis of a two-surgeon consecutive series of DES polyethylene bearing exchanges in a single tertiary referral center. All DESs that subsequently underwent bearing exchange were performed in the same center by one of three fellowship-trained elbow surgeons. All patients were enrolled in long-term surveillance of their elbow arthroplasties; the surveillance encompassed clinical and radiographic review 1, 2, 3, 5, 8 and 10 years postoperative. Data pertaining to bearing exchange indication and time from implantation of DES arthroplasty to bearing exchange were recorded.
Outcome measures were recorded at all surveillance appointments (1, 2, 3, 5, 8 and 10 years postoperative) and included elbow range of movement, Oxford Elbow Score (OES), Mayo Elbow Performance Score (MEPS), requirement for revision surgery and occurrence of complications. Range of motion was measured by a specialist physiotherapist using a goniometer and is reported with standard deviation. Most recent outcome measures are reported following bearing exchange as patients are re-enrolled in long-term surveillance with the follow-up regimen.
Statistical Analysis
Statistical analysis of time to bearing exchange was performed using SPSS ver. 17.0 (SPSS Inc.) and a two-sample Student t-test assuming unequal variances. Statistical significance was set at 5%.
RESULTS
Eleven DES polyethylene bearing exchanges have been attempted in our center in 10 patients with minimum 1-year postoperative follow-up. This procedure was first attempted in our center in July 2015. Patient demographics and elbow status history are presented in Table 1. Indications were polyethylene bearing wear encountered during revision of an aseptically loose humeral component (n=5), bearing failure (n=4) and acute deep infection amenable to treatment with DAIR (n=2). Mean time from primary implantation of the DES arthroplasty to bearing exchange was 5 years, 9 years and 1 month, respectively. There was a significant difference in time from implantation of DES arthroplasty to bearing exchange for revision of aseptically loose humeral component and bearing failure cases (5 years vs. 9 years, respectively; P<0.003). Mean patient age at the time of bearing exchange was 67 years, 68 years and 79 years, respectively.
In situ DES arthroplasty requiring bearing exchange was the primary arthroplasty in eight cases. Primary arthroplasty indications were rheumatoid arthritis (n=2), osteoarthritis (n=2), acute trauma (n=2), posttraumatic arthrosis (n=1), and hemophilic arthropathy (n=1). Bearing exchange was performed to three revision DES arthroplasties. All revision DES arthroplasties were implanted to address a failed Souter-Strathclyde prosthesis. Bearing exchange was performed successfully on first attempt in 10 cases. One case required a second attempt due to a delay in transfer of the bearing from freezer to surgeon. No malpositioned implants were identified. No ulnar ring damage was encountered that may have precluded exchange; therefore, no ulnar component revisions were required.
Clinical Outcomes
One patient (case 2, Table 1) subsequently developed deep infection postoperatively that required treatment with two-stage revision. This case had involved bearing exchange concurrently with revision of an aseptically loose revision humeral prosthesis. This patient was the bilateral case in our series (case 3, Table 1) with both elbows having previous failed Souter-Strathclyde prostheses prior to revision by primary DES. This patient had no significant medical comorbidities. For the remaining 10 bearing exchange DESs, mean follow-up was 3 years (range, 1–7 years). One patient died 3 years following exchange. Of these, no patients required further surgery, and there was no infection recurrence in DAIR cases. No complications occurred. Both DAIR cases involved acute staphylococcus aureus infection and were managed as per BESS guidelines [5]. Both cases received rifampicin for 3 months postoperatively, paired with ciprofloxacin in one case and flucloxacillin in the other.
For all bearing exchange DESs, mean elbow flexion-extension and pronosupination arcs were 107° (±22°) and 140° (±26°), respectively. Mean OES was 36/48 (±12) and MEPS was 83/100 (±19). For bearing exchange performed during revision of an aseptically loose humeral component, mean elbow flexion-extension and pronosupination arcs were 113° (±25°) and 126° (±34°), respectively. Mean OES was 41/48 (±2) and MEPS was 89/100 (±14). For bearing exchange performed for bearing failure, mean elbow flexion-extension and pronosupination arcs were 116° (±18°) and 141° (±17°), respectively. Mean OES was 29/48 (±16) and MEPS was 70/100 (±26). For bearing exchange performed for acute infection, mean elbow flexion-extension and pronosupination arcs were 78° (±4°) and 163° (±11°), respectively. Mean OES was 41/48 (±1) and MEPS was 90/100 (±7). These clinical outcomes, with accompanying median and range values, are presented in Table 2.
DISCUSSION
This is the first series reporting surgeon experience and clinical outcomes in performing DES polyethylene bearing exchange. Our experience demonstrates that the procedure was successful in all cases with only one case requiring use of a second bearing revision kit. United Kingdom (UK) National Joint Registry (NJR) Annual Reports suggest ongoing DES implantation in the UK. The NJR 2017 Annual Report outlined registered UK elbow arthroplasties from 01 April 2012 to 31 December 2017 and included 500 DES implantations [9]. The NJR 2022 annual report included 904 DES implantations, revealing that 404 registered DES implantations were performed in the 4 years between 31 December 2017 and 31 December 2021 [10]. The NJR 2022 Annual Report states that the DES 6-year revision rate is 7.49% (5.66-9.88%) 10 and that 204 of 3,614 confirmed registered total elbow arthroplasties required revision. In total, 76% (n=156) of revision indications were aseptic loosening or infection, scenarios in which polyethylene bearing exchange may be indicated in DES revision.
There are eight published series [2,7,11-16] reporting surgeon experience and clinical outcomes in performing DES arthroplasty that encompass 531 implantations. However, none report surgeon experience or clinical outcomes in performing DES polyethylene bearing exchange. Only one published series related to the DES references polyethylene bearing exchange. Hastings et al. [2], in their prospective multicenter clinical study of 46 DESs that involved a design surgeon, reported that a pin and bearing were replaced in one elbow due to loosening after multiple falls; and treatment of one elbow with a deep infection included condyle and bearing exchange. However, the surgeon experience and clinical outcomes in these cases were not reported.
We report three distinct indications for DES polyethylene bearing exchange: bearing failure, acute infection and a worn bearing encountered during revision of an aseptically loose humeral component. This final indication had not been previously reported. Furthermore, this represents the first series featuring successful DES polyethylene bearing exchange from a non-design surgeon and demonstrates that the procedure is consistent and reproducible when performed by an experienced elbow surgeon.
Our series identifies a significant difference in time from implantation of DES arthroplasty to bearing exchange for revision of an aseptically loose humeral component and bearing failure cases. Our results suggest that the DES ArCom polyethylene bearing may develop symptomatic failure around 9 years postoperatively in the context of a well-fixed adequately positioned prosthesis. Bearing exchange offers a treatment to improve symptoms and prevent catastrophic failure.
Our experience suggests some wear of the polyethylene bearing may be present from 5 years postoperatively. Therefore, we would anticipate a requirement for a polyethylene bearing exchange in revision of an aseptically loose humeral component in a DES that had been implanted greater than 5 years previously. Clinical outcomes achieved are comparable to our previously reported mid- and long-term outcomes in performing a primary DES (mean elbow flexion-extension and pronosupination arcs 115° and 150°, respectively; median OES 40/48, median MEPS 95/100) [7]. No marked deficits in range of motion were observed in this patient cohort even though OES and MEPS results were inferior. However, our cohort’s MEPS score is consistent with mean outcomes reported in systematic review of outcomes after revision total elbow arthroplasty (MEPS of 80 from 21 series including 532 cases) [17].
The indication for bearing exchange may have influenced patient outcomes; however, interpretation should be cautious due to small patient numbers. The mean elbow flexion-extension arc appears to be reduced in cases performed for acute infection. Similar findings were reported by Kwak et al. [18] who, in their comparison of clinical results of revision total elbow arthroplasty for infected and non-infected total elbow arthroplasty, reported that mean ROM arc for flexion-extension was 89.4° and 108°, respectively.
Despite this, both patients treated with bearing exchange DAIR had satisfactory PROM outcomes with no evidence of infection recurrence in either patient. This reinforces the importance of adherence to BESS guidelines [5] in managing periprosthetic elbow infection. Consequently, we recommend that all units utilizing DES arthroplasty should have expertise in performing a polyethylene bearing exchange in case the requirement for a DAIR procedure arises. Our experience is that DES polyethylene bearing exchange is consistently achievable with our outlined surgical technique. We have established that freezer location and the efficiency by which a frozen bearing revision kit is transferred to the operating surgeon are important. One of our cases required the use of a second bearing revision kit due to a delay in transferring the first. This delay resulted in polyethylene bearing thawing with sufficient expansion to prevent insertion into the ulnar component.
We recommend that the operating surgeon personally review the bearing revision kits in the freezer to confirm suitability and that the time-critical nature of the procedure is discussed at the surgical team briefing prior to commencing the operating list. A nominated theatre support worker should be identified for retrieving the bearing revision kit from the freezer when requested by the operating surgeon.
We and the implant manufacturers recommend having two frozen suitable bearing revision kits available for a DES polyethylene bearing exchange. We also recommend ensuring the expertise, equipment and implants are available to perform component revision if a component were malpositioned, damaged or loose or if insertion of both bearings into the ulnar component is not possible. The relative scarcity in performing DES polyethylene bearing exchange has created limitations in our series patient numbers. Collaboration with other centers to increase case numbers was considered, but we were unable to identify a high-volume DES center with an equivalent follow-up regimen. Furthermore, to provide maximum patient numbers in our series we utilized a minimum 1-year follow-up in the inclusion criteria. This relatively short follow-up period for an arthroplasty series may result in failure to identify long-term complications such as subsequent stem loosening or requirement for repeat bearing exchange, though neither occurred in our patient with the longest postoperative follow-up (over 7 years).
CONCLUSIONS
Given the paucity of reports on surgeon experience or clinical outcome, this series provides surgeons managing DES arthroplasty with management principles, successful and reproducible surgical techniques, and expected clinical outcomes when performing DES polyethylene bearing exchange.
Notes
Author contributions
Conceptualization: DLJM. Data curation: DLJM, KW, LP, MM. Formal Analysis: DLJM. Investigation: TC, MPE. Methodology: DLJM. Writing – original draft: DLJM. Writing – review & editing: AAT, DIC, TC, MPE.
Conflict of interest
None.
Funding
None.
Data availability
Contact the corresponding author for data availability.
Acknowledgments
None.