|
 |
- Search
| Clin Shoulder Elb > Volume 27(1); 2024 > Article |
|
Abstract
Background
Rotator cuff tears are often associated with synovitis, but the ability of noninvasive ultrasonography to predict the severity of synovitis remains unclear. We investigated whether ultrasound parameters, namely peak systolic velocity in the anterior humeral circumflex artery and Doppler activity in the glenohumeral joint and subacromial space, reflect synovitis severity.
Methods
A total of 54 patients undergoing arthroscopic rotator cuff repair were selected. Doppler ultrasound was used to measure peak systolic velocity in the anterior humeral circumflex artery and Doppler activity in the glenohumeral joint and subacromial space, and these values were compared with the intraoperative synovitis score in univariate and multivariate analyses.
Results
Univariate analyses revealed that tear size, peak systolic velocity in the anterior humeral circumflex artery, and Doppler activity in the glenohumeral joint were associated with synovitis in the glenohumeral joint (P=0.02, P<0.001, P=0.02, respectively). In the subacromial space, tear size, peak systolic velocity in the anterior humeral circumflex artery, and Doppler activity in the subacromial space were associated with synovitis severity (P=0.02, P<0.001, P=0.02, respectively). Multivariate analyses indicated that tear size and peak systolic velocity in the anterior humeral circumflex artery were independently associated with synovitis scores in both the glenohumeral joint and the subacromial space (all P<0.05).
Rotator cuff tears (RCTs) are a common cause of shoulder pain and dysfunction. Among the most common pathologies encountered during arthroscopic surgery in patients with RCTs is synovitis of the glenohumeral joint (GHJ) and subacromial space (SAS). In recent years, intraoperative grading systems for synovitis haves been established [1,2], and synovitis grade has been shown to have clinical relevance in contributing to pain and dysfunction in patients with RCTs [3-5].
Researchers have begun to investigate whether synovitis in the GHJ is a source of pain in RCT patients; to do this, however, the severity of the synovitis needs to be assessed [6,7]. One study using pulse Doppler showed that peak systolic velocity (PSV) in the anterior humeral circumflex artery (AHCA) was positively associated with intraoperative synovitis severity [6]. Another study showed that Doppler activity in the rotator interval was positively correlated with synovitis severity in patients with partial thickness RCTs [7]. However, more research is required to determine the utility of ultrasound assessment in assessing synovitis severity. The development of a noninvasive ultrasound technique to evaluate synovitis severity would be clinically beneficial in guiding treatment decisions.
The purpose of this study was to determine whether parameters obtained from noninvasive Doppler ultrasound examination reflect intraoperative synovitis severity and to examine the relationship between patient-related factors and synovitis severity. We hypothesized that PSV in the AHCA and power Doppler activity in the GHJ and SAS would correlate with synovitis severity.
This was a retrospective, cross-sectional study conducted at a single primary care facility in a rural area of Japan. This study received approval from the local ethics committee (No. 23C0001) and was conducted following the tenets of the Declaration of Helsinki. The need for written informed consent was waived due to the retrospective study design, and data were anonymized.
A total of 61 consecutive patients with arthroscopic rotator cuff repair between April 2020 and March 2023 were considered eligible for inclusion in the study. Inclusion criteria were as follows: (1) patients with arthroscopic rotator cuff repair, subscapularis tendon intact; (2) available medical records; and (3) available arthroscopic findings. Exclusion criteria were (1) a history of previous shoulder surgery; (2) inflammatory arthritis; and (3) subscapularis tendon tear. After application of the exclusion criteria (previous surgery, n=3; inflammatory arthritis, n=0; subscapularis tendon tear, n=4), 54 patients were selected. The appropriate sample size was calculated to be 47 cases, indexed by the correlation coefficient between PSV in the AHCA and intra-articular inflammatory findings reported in a previous study (r=0.40 [6], alpha=0.05, power=0.80). Therefore, these 54 patients were analyzed (Fig. 1).
Two days before surgery, we used ultrasound (3–11 MHz linear probe, SONIMAGE HS2, Konica Minolta) to measure two parameters: PSV and power Doppler activity. PSV in the AHCA was measured using a method that is recognized as highly reliable (intraobserver reproducibility, 0.983–0.996; interobserver reproducibility, 0.949–0.985) [6,8,9], and we also confirmed high intraobserver reproducibility in the pretest before data collection (0.986; 95% confidence interval, 0.942–0.996). Briefly, the patient was seated in a chair with the shoulder joint abducted at 30°, the elbow joint flexed at 90°, and the hand placed palm-up on the armrest. The bicipital groove and AHCA were identified by transverse scanning, then blood flow velocity in the AHCA was measured by longitudinal scanning, and the PSV was calculated by pulse Doppler ultrasonography (Fig. 2) [9].
Power Doppler activity was evaluated with binary presence/absence values. Similar methods have been applied to knee osteoarthritis [10,11] with high reliability (intraobserver reproducibility, 0.80; interobserver reproducibility, 0.62) [12], and we confirmed a high intraobserver reproducibility of 0.886 (95% confidence interval, 0.710–0.922). These measurements were taken at the anterior part of the lateral GHJ capsule [13] and the SAS [14], which receives blood from the AHCA [15,16]. Briefly, in the same position as the PSV measurement described above, the probe was adjusted vertically to visualize the GHJ capsule using the lesser tuberosity and insertion site of the subscapularis tendon as landmarks according to the method reported by Alilet et al. [13] (Fig. 3A). For the SAS, the probe was placed on the coracoacromial ligament and its deep subacromial bursa was visualized using the acromion and coracoid processes as landmarks [14] (Fig. 3B). All ultrasound examinations were performed by an independent blinded examiner.
Retrospectively, GHJ [1] and SAS [2] inflammation were graded on video during arthroscopic surgery by an independent, blinded examiner. The GHJ synovitis grading system of Davis et al. [1] was used considering the following variables: color of anterior capsule (pale=0, pink=1, or red=2); villous projections (none=0, few=1, or extensive=2); capillaries in the capsule (scattered=0 or hypertrophied =1); and axillary recess (normal=0 or contracted =1). Total GHJ synovitis scores ranged from 0 to 6, with a higher score indicating a greater degree of GHJ synovitis (Fig. 4A). According to the SAS synovitis grading system proposed by Jo et al. [2], grading was as follows: size of the synovial villi (<2 mm=0, 2 to 5 mm=1, or >5 mm=2); redness of the villi (pale=0, slightly reddish=1, or definitely red=2); and density of synovial villi in the relevant region (<1/3=0, ≥1/3=1). Note that only the region that receives blood supply from the AHCA [16], the anterior half of the "lateral subacromial synovium" as classified by Jo et al. [2], was evaluated in this study. Total SAS synovitis scores ranged from 0 to 5, with a higher score indicating a greater degree of SAS synovitis (Fig. 4B).
Available clinical parameters included age, sex, side, duration of symptoms, history of trauma, diabetes, biceps tendon disease, muscle atrophy, fatty infiltration, and tear size. Muscle atrophy was graded according to the Thomazeau classification [17], fatty infiltration according to Goutallier classification [18], and tear size according to Cofield classification [19]. Furthermore, the use of non-steroidal anti-inflammatory drugs and corticosteroid injections was investigated.
First, univariate analysis was performed to identify ultrasound parameters (PSV in the AHCA, Doppler activity in the GHJ and SAS) and patient-related factors associated with synovitis scores in the GHJ and SAS, and the effect size, r, was calculated. Continuous scores were compared by Pearson correlation test, ordinal scores by Spearman correlation test, and dichotomous scores by independent t-test. Subsequently, factors significantly associated with synovitis scores in the univariate analysis were included in the multivariate analysis. A variance inflation factor of less than 10 was considered statistically acceptable. Statistical significance was accepted at P<0.05. All statistical analyses were performed with R version 3.4.1 (R Foundation for Statistical Computing).
Table 1 shows the clinical characteristic parameters of the 54 patients with a mean age of 68 years (standard deviation [SD], 8 years; 29 males and 25 females). All patients were administrated nonsteroidal anti-inflammatory drugs (celecoxib, 60 mg) the day before surgery. None of the patients received corticosteroid injections in the 6 months before surgery. Mean (SD) total synovitis score in the GHJ was 2.54 (1.82), and that in the SAS was 2.15 (1.66). Mean PSV in the AHCA was 17.8 cm/sec (9.5 cm/sec). The number of times Doppler activity was detected in the GHJ was 13 (24.0%). In the SAS, Doppler activity was detected in 27 cases (50.0%). Tear size, PSV in the AHCA, and Doppler activity in the GHJ were significantly associated with GHJ synovitis scores (P=0.02, P<0.001, P=0.02, respectively). Tear size, PSV in the AHCA, and Doppler activity in the SAS were significantly associated with SAS synovitis scores (P=0.02, P<0.001, P=0.02, respectively) (Table 2). No associations were found between other clinical parameters and synovitis scores.
Multivariate analyses revealed that PSV in the AHCA and tear size were significantly associated with GHJ synovitis score (P=0.01, P=0.04, respectively), as they also were with the SAS synovitis score (P<0.001, P=0.003, respectively) (Table 3). All values of the variance inflation factor were statistically acceptable.
The main findings of this study are that higher PSV in the AHCA was independently associated with more severe synovitis in the GHJ and SAS; the presence of Doppler activity in the GHJ and SAS was correlated with synovitis but was not an independent indicator; and large tear size was independently associated with more severe synovitis. These findings suggest that PSV in the AHCA and tear size, both of which can be measured noninvasively, may be useful indicators of synovitis severity.
As expected, the results showed that PSV in the AHCA correlated with synovitis in the GHJ, which is consistent with the findings of a previous study [6]. Furthermore, our results showed that PSV in the AHCA correlated with synovitis in the SAS, which is a novel finding. Several previous studies evaluated synovitis in the GHJ and SAS separately [3,20] and showed that the effect on pain and function differed according to the degree of synovitis in these two sites. Therefore, it would be clinically relevant to evaluate and analyze GHJ and SAS synovitis severity separately.
Our findings and previous research suggest that PSV in the AHCA is a clinically useful parameter because it has high intra- and inter-rater reliability [6,8,9], is associated with histologic synovitis scores as well as macroscopic findings [6], is easily identifiable due to clear bony landmarks [8], and is less susceptible to probe compression due to the presence of the artery under the transverse ligament [8]. Therefore, PSV is a clinically useful indicator of synovitis that can be measured noninvasively.
We observed a correlation between Doppler activity in the GHJ and SAS with synovitis in these respective locations. These findings partially differ from a previous power Doppler study [7], which found a correlation between Doppler activity with synovitis in the rotator interval but not in the SAS. This discrepancy may be due to differences in the measurement position, as Inoue et al. [7] suggested that the SAS be measured during shoulder extension, resulting in microvessel loss due to compression. In our study, we examined the SAS with the shoulder joint at 30° of abduction [9], viewed over the coracoacromial ligament, to minimize compression. Conversely, the rotator interval in the previous study and where we measured GHJ reflect the same branches of the AHCA [16], resulting in consistent findings. Furthermore, the confounding factor of synovitis in the anterior part of the GHJ was minimized in our study as we excluded patients with subscapularis tendon tears. Therefore, it is plausible that Doppler activity in the GHJ and SAS is associated with synovitis.
However, multivariate analysis did not extract Doppler activity as an independent indicator of synovitis. Furthermore, Doppler activity had a smaller (although significant) effect size on synovitis than PSV in the AHCA. PSV in the AHCA therefore is a stronger indicator of synovitis severity than Doppler activity. When applying Doppler activity to patients with RCTs, the following considerations should be kept in mind: (1) power Doppler may be less frequently observed in non-rheumatoid shoulder diseases [21,22], and it is a sensitive marker only during the active inflammatory phase [12,23]; (2) differences in measurement position can lead to errors in power Doppler findings [7]; and (3) PSV is quantitative, whereas Doppler activity is semiquantitative [6]. Nevertheless, the possibility that Doppler activity may be indicative of synovitis cannot be ruled out, and a system for assessing Doppler activity with high accuracy warrants future research.
We found that larger tear size was independently associated with more severe synovitis, consistent with previous studies [24-26]. Full-thickness RCTs have been associated with elevated synovitis levels compared to both control groups [24] and partial-thickness tears [25]. Moreover, a correlation between synovitis severity and tear size has previously been reported [26]. However, it is important to note that our current study could not establish a causal relationship between synovitis and the progression of RCTs due to its retrospective nature. This should be investigated in future studies.
Numerous previous studies have reported that synovitis is often observed intraoperatively in patients with RCTs [1-7], highlighting the need for noninvasive assessment of synovitis. Our study findings suggest that a simple noninvasive test may assist clinicians in assessing the severity of synovitis in patients with RCTs, potentially refining treatments to improve outcomes. As our focus was primarily on establishing correlations between PSV in the AHCA and synovitis severity, we did not assess pain or function. However, we recognize that clinical outcomes such as pain and function are critical in assessing the impact of synovitis on patient quality of life [3]. We are conducting ongoing clinical trials that include synovitis, PSV in the AHCA, pain, and function, which will provide comprehensive clinical insights.
This study has several limitations. First, we included patients from a single primary care facility in a rural area of Japan, which could have introduced selection bias. Differences between urban and rural areas [27] or research settings [28] may lead to differences in patient severity, and therefore, generalization of our findings should be made with caution. Second, only the area supplied by the AHCA was evaluated. The shoulder is also supplied by the thoracoacromial, posterior humeral circumflex, scapular circumflex, and axillary arteries [15,16]. However, several studies examining each site of the GHJ in detail have shown that anterosuperior synovitis is usually greater than posterior and inferior synovitis [2,4], therefore measuring PSV in the AHCA and its supply regions is reasonable and valid. Finally, histologic evaluation was not performed in this study. Rather, we focused on ultrasound imaging and arthroscopic findings, which we believe provide clinically relevant findings. Further investigations, including histologic evaluation, are warranted to expand our understanding.
The current study showed that greater PSV in the AHCA and larger tear size were independently associated with the degree of synovitis in the GHJ and SAS. These findings suggest that PSV in the AHCA and tear size, both of which can be measured noninvasively, are useful indicators of synovitis severity.
NOTES
Fig. 2.
Measurement of peak systolic velocity in the anterior humeral circumflex artery. (A) Bicipital groove transverse color Doppler ultrasonography. The anterior humeral circumflex artery is indicated by the arrow. (B) Longitudinal view of the anterior humeral circumflex artery in a pulsed Doppler. The arrow indicates peak systolic velocity in the anterior humeral circumflex artery.

Fig. 3.
Evaluation of Doppler activity. (A) The anterior part of the glenohumeral joint capsule at the insertion of the subscapularis tendon. (B) The subacromial bursa deep to the coracoacromial ligament. SSC: subscapularis tendon, LT: lessor tuberosity, C: coracoid process, A: acromion process.
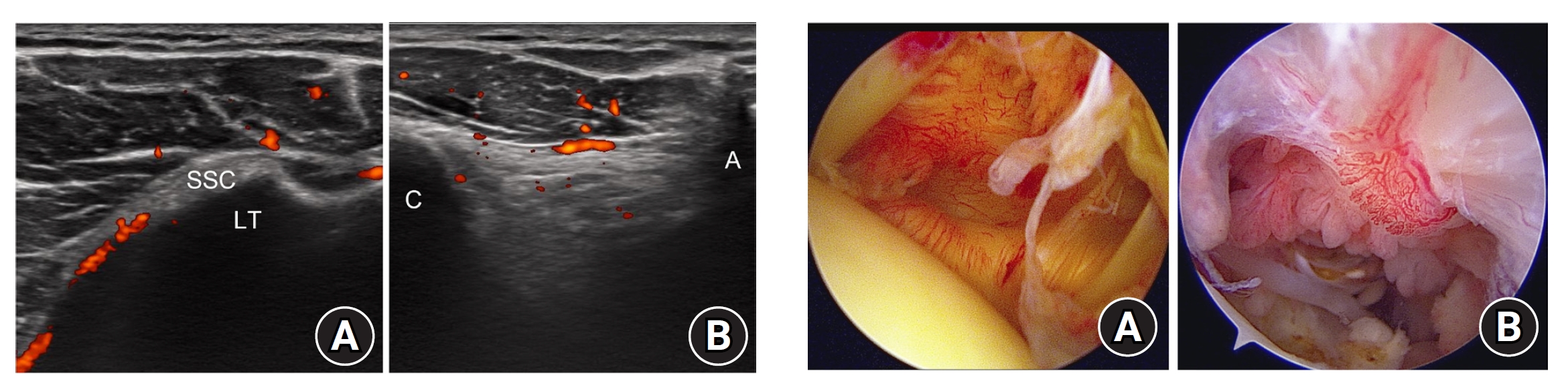
Fig. 4.
Arthroscopic images of the glenohumeral joint capsule and subacromial space. (A) Synovitis in the glenohumeral joint. The capsule was pink, there were few villous projections, capillaries in capsule were hypertrophied, and the axillary recess was contracted. This corresponds to a Davis grade of 4. (B) Synovitis in the subacromial space. Hypertrophy of the synovial villi was more than 5 mm, villi were slightly reddish, and the density of synovial villi was ≥1/3. This corresponds to a Jo grade of 4.
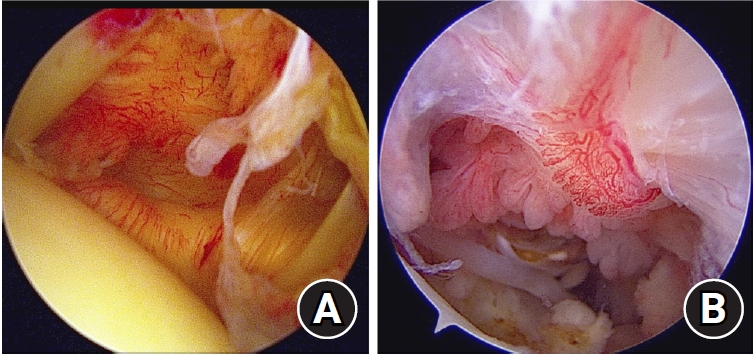
Table 1.
Patient characteristics
Table 2.
Univariate analysis of synovitis scores and clinical parameters
| Parameter |
GHJ |
SAS |
||||
|---|---|---|---|---|---|---|
| t-value | r | P-value | t-value | r | P-value | |
| Age | 0.082 | 0.01 | 0.93 | 0.087 | 0.01 | 0.93 |
| Sex | –0.231 | –0.03 | 0.82 | 0.275 | 0.04 | 0.78 |
| Dominant | –0.387 | –0.05 | 0.70 | –1.939 | –0.26 | 0.06 |
| Duration | –0.822 | –0.11 | 0.42 | –0.478 | –0.07 | 0.64 |
| Trauma | –0.051 | –0.01 | 0.96 | 0.469 | 0.06 | 0.64 |
| Diabetes | –0.381 | 0.05 | 0.70 | 0.474 | 0.07 | 0.64 |
| Biceps tendon disease | 1.626 | 0.22 | 0.11 | 0.945 | 0.13 | 0.35 |
| Muscle atrophy | 1.908 | 0.26 | 0.06 | 1.958 | 0.27 | 0.05 |
| Fatty infiltration | 1.908 | 0.26 | 0.06 | 1.932 | 0.26 | 0.06 |
| Tear size | 2.323 | 0.31 | 0.02* | 2.472 | 0.33 | 0.02* |
| PSV in the AHCA | 3.800 | 0.47 | <0.001* | 5.159 | 0.58 | <0.001* |
| Doppler activity in GHJ | 2.346 | 0.31 | 0.02* | NA | NA | NA |
| Doppler activity in SAS | NA | NA | NA | 2.371 | 0.31 | 0.02* |
Table 3.
Multivariate analysis of synovitis and clinical parameters
| Variable |
GHJ |
SAS |
||||||
|---|---|---|---|---|---|---|---|---|
| t-value | β | P-value | VIF | t-value | β | P-value | VIF | |
| PSV in the AHCA | 2.647 | 0.36 | 0.01* | 1.34 | 3.859 | 0.48 | <0.001* | 1.38 |
| Doppler activity in GHJ | 1.439 | 0.20 | 0.16 | 1.36 | NA | NA | NA | NA |
| Doppler activity in SAS | NA | NA | NA | NA | 1.585 | 0.21 | 0.12 | 1.53 |
| Tear size | 2.151 | 0.26 | 0.04* | 1.34 | 3.082 | 0.34 | 0.003* | 1.10 |
REFERENCES
1. Davis DE, Maltenfort M, Abboud JA, Getz C, Rothman Institute Shoulder Consortium Group and the Association of Clinical Elbow and Shoulder Surgeons. Classifying glenohumeral synovitis: a novel intraoperative scoring system. J Shoulder Elbow Surg 2017;26:2047–53.


2. Jo CH, Shin JS, Kim JE, Oh S. Macroscopic and microscopic assessments of the glenohumeral and subacromial synovitis in rotator cuff disease. BMC Musculoskelet Disord 2015;16:272.



3. Kim DH, Bae KC, Choi JH, Na SS, Hwang I, Cho CH. Chronicity is associated with the glenohumeral synovitis in patients with a rotator cuff tear. J Orthop Res 2021;39:2226–33.


4. Lee D, Lee KH, Jo YH, et al. Correlation between severity of synovitis and clinical features in rotator cuff tears. Clin Orthop Surg 2021;13:88–96.



5. Tan Z, Hendy BA, Zmistowski B, et al. Glenohumeral synovitis score predicts early shoulder stiffness following arthroscopic rotator cuff repair. J Orthop 2020;22:17–21.



6. Asano H, Terabayashi N, Kawashima K, et al. Blood flow in the anterior humeral circumflex artery reflects synovial inflammation of the shoulder joint in rotator cuff tears. JSES Int 2022;6:623–30.



7. Inoue A, Funakoshi T, Koga R, et al. Evaluation of hypervascularity in synovitis of the shoulder using ultrasound: comparison of preoperative ultrasound findings and intraoperative arthroscopic findings. JSES Int 2022;6:473–8.



8. Terabayashi N, Watanabe T, Matsumoto K, et al. Increased blood flow in the anterior humeral circumflex artery correlates with night pain in patients with rotator cuff tear. J Orthop Sci 2014;19:744–9.


9. Watanabe A, Katayama H, Machida T, Hirooka T. Synchronization of blood flow velocity in the anterior humeral circumflex artery and reduction in night pain after arthroscopic rotator cuff repair: a case report. Cureus 2022;14:e24468



10. Bruyn GA, Naredo E, Damjanov N, et al. An OMERACT reliability exercise of inflammatory and structural abnormalities in patients with knee osteoarthritis using ultrasound assessment. Ann Rheum Dis 2016;75:842–6.


11. Oo WM, Linklater JM, Hunter DJ. Imaging in knee osteoarthritis. Curr Opin Rheumatol 2017;29:86–95.


12. Oo WM, Linklater JM, Bennell KL, et al. Are OMERACT Knee Osteoarthritis Ultrasound Scores associated with pain severity, other symptoms, and radiographic and magnetic resonance imaging findings. J Rheumatol 2021;48:270–8.


13. Alilet M, Behr J, Nueffer JP, Barbier-Brion B, Aubry S. Multi-modal imaging of the subscapularis muscle. Insights Imaging 2016;7:779–91.



14. Wu CH, Chang KV, Su PH, Kuo WH, Chen WS, Wang TG. Dynamic ultrasonography to evaluate coracoacromial ligament displacement during motion in shoulders with supraspinatus tendon tears. J Orthop Res 2012;30:1430–4.


15. Põldoja E, Rahu M, Kask K, Bulecza T, Weyers I, Kolts I. Blood supply of the superior glenohumeral ligament: a gross anatomical and histological study. Austin J Anat 2016;3:1048.
16. Põldoja E, Rahu M, Kask K, Weyers I, Kolts I. Blood supply of the subacromial bursa and rotator cuff tendons on the bursal side. Knee Surg Sports Traumatol Arthrosc 2017;25:2041–6.


17. Thomazeau H, Boukobza E, Morcet N, Chaperon J, Langlais F. Prediction of rotator cuff repair results by magnetic resonance imaging. Clin Orthop Relat Res 1997;(344):275–83.

18. Goutallier D, Postel JM, Gleyze P, Leguilloux P, Van Driessche S. Influence of cuff muscle fatty degeneration on anatomic and functional outcomes after simple suture of full-thickness tears. J Shoulder Elbow Surg 2003;12:550–4.


19. Cofield RH, Parvizi J, Hoffmeyer PJ, Lanzer WL, Ilstrup DM, Rowland CM. Surgical repair of chronic rotator cuff tears: a prospective long-term study. J Bone Joint Surg Am 2001;83:71–7.

20. Cho CH, Bae KC, Kim DH. Incidence and risk factors for early postoperative stiffness after arthroscopic rotator cuff repair in patients without preoperative stiffness. Sci Rep 2022;12:3132.



21. Park EW, Cho JH, Cho CH, Sung DH, Kim DH. Comparison of shoulder ultrasonographic assessments between polymyalgia rheumatica and frozen shoulder in patients with bilateral shoulder pain: a comparative retrospective study. J Pers Med 2021;11:372.



22. Suzuki T, Yoshida R, Okamoto A, Seri Y. Semiquantitative evaluation of extrasynovial soft tissue inflammation in the shoulders of patients with polymyalgia rheumatica and elderly-onset rheumatoid arthritis by power Doppler ultrasound. Biomed Res Int 2017;2017:4272560.



24. Abrams GD, Luria A, Carr RA, Rhodes C, Robinson WH, Sokolove J. Association of synovial inflammation and inflammatory mediators with glenohumeral rotator cuff pathology. J Shoulder Elbow Surg 2016;25:989–97.


25. Shindle MK, Chen CC, Robertson C, et al. Full-thickness supraspinatus tears are associated with more synovial inflammation and tissue degeneration than partial-thickness tears. J Shoulder Elbow Surg 2011;20:917–27.



26. Gotoh M, Hamada K, Yamakawa H, et al. Interleukin-1-induced subacromial synovitis and shoulder pain in rotator cuff diseases. Rheumatology (Oxford) 2001;40:995–1001.


- TOOLS