Complications of reverse shoulder arthroplasty: a concise review
Article information
Abstract
Reverse shoulder arthroplasty is an ideal treatment for glenohumeral dysfunction due to cuff tear arthropathy. As the number of patients treated with reverse shoulder arthroplasty is increasing, the incidence of complications after this procedure also is increasing. The rate of complications in reverse shoulder arthroplasty was reported to be 15%–24%. Recently, the following complications have been reported in order of frequency: periprosthetic infection, dislocation, periprosthetic fracture, neurologic injury, scapular notching, acromion or scapular spine fracture, and aseptic loosening of prosthesis. However, the overall complication rate has varied across studies because of different prosthesis used, improvement of implant and surgical skills, and different definitions of complications. Some authors included complications that affect the clinical outcomes of the surgery, while others reported minor complications that do not affect the clinical outcomes such as minor reversible neurologic deficit or minimal scapular notching. This review article summarizes the processes related to diagnosis and treatment of complications after reverse shoulder arthroplasty with the aim of helping clinicians reduce complications and perform appropriate procedures if/when complications occur.
INTRODUCTION
Reverse shoulder arthroplasty (RSA) was developed initially as a salvage procedure for cuff tear arthropathy; however, its use has been extended to other shoulder conditions, such as irreparable rotator cuff tear, glenohumeral osteoarthritis, proximal humerus fracture, and failed anatomical shoulder arthroplasty [1]. The surgical outcome of RSA is promising, and the technique has been increasingly used [2,3]. However, with increasing application of RSA, the number of complications has increased, which occasionally requires interventions [3].
The rate of complications with RSA is approximately 15%–24% [3-6]. The complication rate differs among studies because of different definitions of complications and different prostheses used [6]. Some authors reported only major complications that affect the clinical outcome. Other studies reported both major and minor complications, including reversible neurologic deficit and minimal scapular notching [3,6-8].
The incidence of complications has changed over time. According to a systemic review conducted by Zumstein et al. [6] in 2011, the most common complication of RSA is instability (6.9%), followed by infection (5.6%), aseptic glenoid loosening (5.0%), acromion/scapular spine fracture (2.2%), glenoid or humeral disassembly (2.2%), humeral fracture (2.1%), humeral loosening (1.9%), and neurologic complications (1.7%). However, Ascione et al. [5] reported in 2018, a total complication rate of 18.7% in 1,035 cases of RSA in a 5-year follow-up study. They reported that infection (4.1%) was the most common complication, followed by instability (3%), neurologic complications (2.1%), glenoid complications (2.3%), and scapular fractures (1.1%). With improvements in prosthesis design and surgical skills, the rate of infection seems to be outpacing the rate of dislocation after RSA.
In our clinic, 438 RSAs were performed between March 2009 and December 2019, and 40 cases of complications were reported. The total complication rate after RSA was 9.1%. The most common complication was intraoperative humerus fracture (3.2%), followed by Periprosthetic joint infection (1.1%), acromion/scapular spine fracture (1.1%), neurologic complications (0.7%), and dislocation (0.5%). In addition, minor problems including grade 1 or higher scapular notching (32.7%) and stress shielding of humerus (26.3%) were observed in our clinic. The purpose of this article is to describe the complications after RSA with the aim to provide information to help clinicians manage RSA-related complications.
DISLOCATION
Dislocation is a common complication after RSA and requires surgical intervention in the early period (<2 years) [3,5,6,9]. The incidence of dislocation was reported to be 4.7% by Zumstein et al. [6]. A recent systemic review stated that dislocation was the second most common complication [5]. With improvements in RSA prosthesis design and surgical skills, the incidence of early dislocation after RSA seems to be decreasing. However, dislocation remains a difficult complication to correct because of the high failure rate after reoperation. Chalmers et al. [10] reported that 85% of primary RSA cases and more than 50% of revision RSA cases had successful outcomes after revision surgery.
There are multiple predisposing factors for early dislocation; therefore, it is important to determine the main cause of dislocation before reoperation. Previous surgery, including anatomical total shoulder arthroplasty and hemiarthroplasty, is a risk factor for dislocation after RSA [9,11]. The lack of soft tissue tension due to implant malposition, improper version of implant, and mechanical impingement [9,11,12] as well as subscapularis deficiency in medialized prosthesis design are known risk factors for dislocation [9,11,12]. If early dislocation is caused by improper humeral or glenoid implant version, reoperation must be performed to normalize the implant version [9]. The humeral implant version can be measured by torsional-computed tomography including the elbow joint.
Deltoid tension is increased by glenoid lateralization and humerus distalization [9,11]. In cases of excessive medialization of the center of rotation, lateralization of the glenoid should be performed [9]. When humeral medialization is less than 15 mm, a larger or lateralized glenosphere or lateralizing is a choice [9,13,14]. However, when these options are not sufficient because of severe deficiency of the glenoid bone stock, bony increased offset-reversed shoulder arthroplasty is an option [9,11].
Humeral distalization is determined by humeral length and glenosphere position/size. Humeral length can be shortened if the original length is not restored. In proximal humeral bone loss such as proximal humerus fracture, revision RSA, osteolysis of proximal humerus, or overcutting of the humeral head in primary RSA, restoring the original length is challenging. If humeral height is short compared with the normal opposite side on plain radiograph, it can be increased using a thick polyethylene liner or thick metal tray (Fig. 1) [9,10,14]. However, the typical increase in height is 15–20 mm and differs by prosthesis [9]. If the height reduction exceeds 15–20 mm compared with the humeral length on the opposite side, humeral stem revision for height restoration using a cemented stem or structural humeral bone graft should be considered [3,9]. In addition, using a larger glenosphere and placing a glenosphere inferiorly can generate humerus distalization and increased deltoid tension.
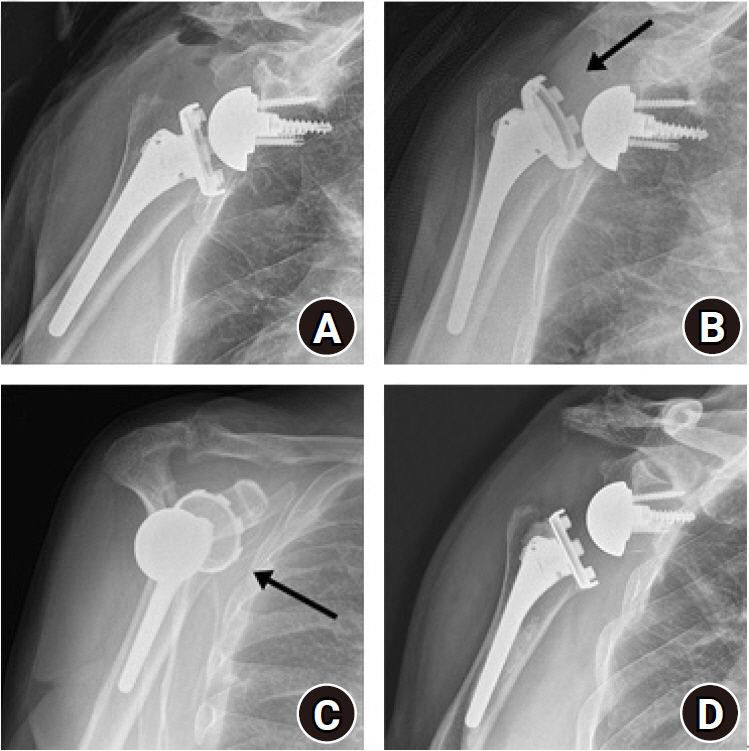
Early dislocation after reverse shoulder arthroplasty (RSA). (A) Anteroposterior (AP) radiograph after primary RSA. AP (B) and scapular (C) scapular Y-views showed anterior dislocation (arrows) of the humeral prosthesis at 4 months after surgery. (D) Revision RSA with polyethylene liner change was performed, and no dislocation had recurred over 2 years of follow-up.
Late dislocation can be caused by a change in implant position. There are many causes of implant position change, such as subsidence or rotation of the humeral stem and baseplate movement. Implant loosening can be detected by serial radiograph during follow-up and can arise from aseptic or septic loosening. Aseptic humeral loosening can be caused by stress shielding of the humerus or polyethylene debris of scapular notching [5]. Cases of glenoid baseplate loosening have been reported; however, in RSA, the rate of aseptic loosening of the baseplate was lower than that of the humeral stem [5,9]. Because of medialization of the glenoid in RSA, torque stress was lower on the glenoid side than on the humeral side [9,15]. In cases of implant loosening by periprosthetic infection, two-stage revision is the treatment of choice.
Subscapularis restoration also affects dislocation. The subscapularis is considered a protector of anterior dislocation in medialized RSA design [9,11,12]. In lateralized RSA design, horizontal deltoid compression stabilizes the shoulder joint; therefore, subscapularis repair is not required to prevent shoulder dislocation [16]. However, a recent meta-analysis showed that subscapularis repair reduces the rate of dislocation regardless of implant design [17]. Surgeons should assess the subscapularis tendon before the operation and consider implant design and position to prevent dislocation after surgery.
PERIPROSTHETIC JOINT INFECTION
Periprosthetic joint infection has been the second most common complication of RSA, with an incidence rate ranging from 1% to 10% [5,6]. However, given the recent decrease in rate of dislocations, infection has become the most common complication [5]. Ascione et al. [5] reported a 4.1% rate of periprosthetic infection, which was the most commonly observed complication in 1,035 RSAs with an at least 5-year follow-up in 2018. In addition, Portillo et al. [18] reported that prosthesis failure within 2 years of implantation is a strong indicator of infection. Also, periprosthetic joint infection is the most common reason for revision arthroplasty within 2 years after RSA [19].
One predisposing factor for infection after RSA is prior shoulder surgery [11]. Previous arthroscopic rotator cuff repair is related to increased infection rates [9,11]. Other predisposing factors include morbid obesity (body mass index >40 mg/m2), uncontrolled diabetes (glucose >200 mg/L, hemoglobin A1C >7%), rheumatoid arthritis, malnutrition, young age (< 65 years), intravenous drug abuse, long operation time (>115 minutes), and number of times the surgical room door was opened during surgery [9,20,21].
Unlike hip and knee arthroplasty, in shoulder arthroplasty, the most commonly isolated organism is Cutibacterium acnes (formerly Propionibacterium acnes) (38.9%) [12,22-24]. This lipophilic and anaerobic non-spore forming Gram-positive rod-shaped bacteria is part of the normal flora of human skin [23-25]. In the deep dermis, the bacteria digests the sebum and secretes free fatty acids on the skin, generating the overall acidic environment of the skin [23]. The bacterial burden of C. acnes is higher in the anterior and posterior acromion and the axilla compared to other skin areas [26]. Men have a higher bacterial burden on the shoulder than women [25].
C. acnes is a slow-growing organism, and it takes 10–14 days to detect positive results from culture [27]. In addition, the bacteria produces biofilms on the body and metal prostheses, disturbing phagocytosis [27]. Patients with C. acnes infection present with unexplained continuous shoulder pain, stiffness, and osteolysis without overt signs of infection, such as swelling, redness, heat sensation, and effusion [23-25,28]. Staphylococcus epidermidis (14.8%) and Staphylococcus aureus (14.5%) are other commonly observed organisms [9,11,12].
Many strategies can be used for prevention of periprosthetic infection. Bathing with chlorhexidine gluconate on the day before surgery reduces the risk of infection [22,29]. Hair shaving before surgery is not necessary [30]. Administration of first-generation cephalosporin as a preventive antibiotic 1 hour before surgery is recommended; however, C. acnes will not be completely eliminated from the surgical field [31]. During skin preparation, chlorhexidine must be allowed to dry completely before draping [32]. Benzoyl peroxide recently has been reported to effectively decrease the burden of C. acnes [24]. Laminar flow in the operating room was ineffective for reducing risk, but reducing the number of times the surgical room doors were opened during surgery helps reduce the risk of infection [33]. Changing surgical gloves regularly, changing the blade after skin incision, frequent surgical site irrigation, irrigation with diluted povidone (1.3 g/L), injection of gentamicin at the time of closure, use of antibiotic-loaded cement (1 g of vancomycin/bone cement), and use of topical adhesives for skin closure were reported to be effective in decreasing the risk of infection after arthroplasty [18-20,34-36].
If patients have purulent joint fluid with pus discharge fistula, diagnosis of periprosthetic joint infection is not difficult. However, in cases of low-grade infection without joint fluid or normal infection markers on laboratory tests, intraoperative biopsy and culture play a crucial role in diagnosis [37]. Tissue biopsy is more accurate than fluid aspiration; biopsy tissue culture has 100% sensitivity and 100% specificity, while aspirate culture has 16.7% sensitivity and 100% specificity [37]. In addition, Hsu et al. [22] recommended harvesting a minimum of five biopsy samples during surgery for C. acnes culture.
The preferred management strategy for infected RSA remains controversial [34]. In cases of acute infection (<6 weeks), open irrigation and debridement with exchange of modular components are regarded as standard treatment [38]. However, the results of this treatment strategy are not conclusive. Ortmaier et al. [36] reported a success rate of 50% (2/4) with prior treatment, and patients with failure required additional surgery. In cases of chronic infection, traditional two-stage revision is the gold standard treatment for periprosthetic joint infection [12,36,39]. Two-stage revision showed an infection recurrence rate of 0%–36% [40]. This strategy shows the best result in terms of eradication of infection, pain relief, and restoration of function but requires a long treatment time (Fig. 2) [41].
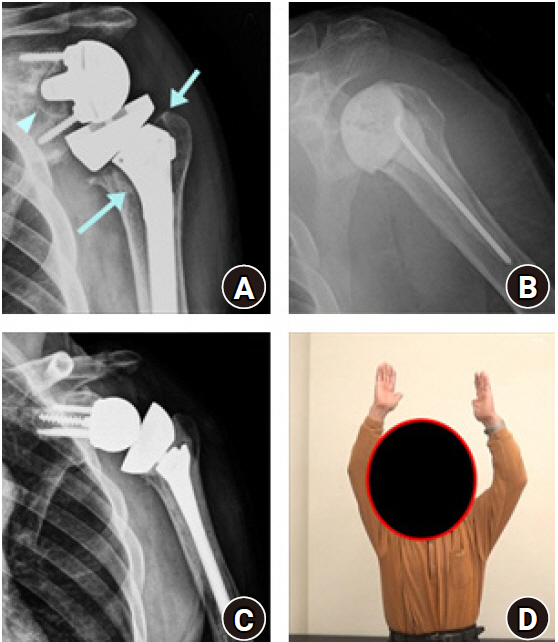
Two-stage revision for infected reverse shoulder arthroplasty (RSA). (A) Anteroposterior radiograph of primary RSA after 18 months. Radiolucency around the humeral stem (arrows) at the metaphysis and glenoid baseplate (arrowhead) was observed. (B) Implant removal and anti-mixed cement spacer insertion was performed. (C) After infection control, revision RSA with cemented humeral stem was performed, and (D) greater than 120˚ of left shoulder elevation was achieved at 2 years after the final surgery (photograph used with permission for study purpose).
Some short-duration treatment procedures have been attempted to reduce treatment time and patient discomfort. Debridement and retention of the prosthesis have been attempted, but have not shown consistent satisfactory results [9,34]. In a French multicenter study, among 17 patients who underwent debridement and partial component retention, 7 showed clearance of infection [42], and Romanò et al. [34] reported unsatisfactory results after component retention. One-stage exchange has been gaining attention for its advantages of reduced dissection length, reduced stress to soft tissues, and reduced time and costs [40]; furthermore, Klatte et al. [43] reported a 94% success rate with a mean follow-up period of 4.7 years. In a systematic review, both one- and two-stage exchange provided greater than 85% eradication rates [41]. In addition, cement spacers could be a long-term treatment option for joints with low functional demands [42,44].
INTRAOPERATIVE FRACTURE
Intraoperative fractures can occur on the humeral or glenoid side during surgery. Both types of fracture are uncommon complications during surgery but are difficult to manage. Boileau et al. [45] reported one case of perioperative humeral fracture and one case of intraoperative glenoid fracture in 45 patients over a mean follow-up of 40 months. In a review article by Zumstein et al. [6], the rate of intraoperative humeral fracture was 2.0% (16/782), and that of intraoperative glenoid fracture was 0.9% (7/782).
Humeral side fractures during surgery are more common than glenoid fractures. A systemic review reported humeral side fractures in 1.8% (91/5,539 shoulders) of patients [3]. In our clinic, intraoperative humeral fracture was observed in 3.2% of 438 RSAs. In particular, if a large press-fit stem (high filling ratio) [46] is used, the proximal humerus rim can be damaged during impaction. In addition, fractures occur frequently during arm positioning, such as extension, rotation, and translation, to dislocate or reduce the humeral head. During arm positioning, spiral fracture of the humeral metaphysis or greater tuberosity avulsion fracture could occur [47]. Other risk factors for intraoperative humeral fracture are osteopenia, rheumatoid arthritis, and revision surgery [47]. To prevent intraoperative humeral fractures, surgeons should be aware of the risk factors for periprosthetic fracture [48]. During humerus positioning, inferior capsular release from the humerus must be carried out. In addition, during implant insertion, broaching should be performed parallel to the humeral shaft, and excessive fitting of the humeral stem should be avoided.
The treatment plan for intraoperative humeral fracture must include fracture site, displacement, bone quality, and stability of the humeral stem. If a displaced fracture occurs before humeral insertion or if impending fracture occurs without displacement, cerclage wiring can help stabilize the fracture site during subsequent procedures [49]. If a fracture occurs after humeral stem insertion, the stability of the humeral stem should be evaluated. In a stable humeral stem, cerclage wiring and additional fixation without stem change are sufficient, while unstable humeral stem requires repair with a long or cemented stem to achieve stability (Fig. 3) [49].
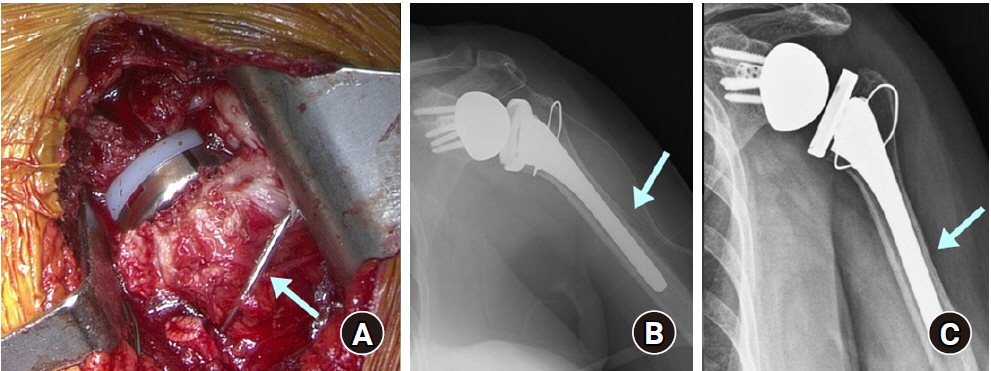
Periprosthetic humeral fracture during reverse shoulder arthroplasty. (A) During surgery, the humeral spiral fracture was stabilized with cerclage wire (arrow), and the humeral stem was inserted with firm fixation. (B) Immediate postoperative anteroposterior radiograph showed fracture around the humeral stem (arrow). (C) The fracture was healed at 4 months after surgery (arrow).
Intraoperative glenoid fractures rarely occur during surgery, with an incidence rate of 0.3% [3]. These fractures can occur during the reaming procedure or fixation of glenoid implants. In patients with osteoporosis, care must be taken during reaming and impaction of the baseplate. In addition, in patients with degenerative osteoarthritis, the glenoid can be fractured despite a high level of hardness [50]. This can be caused by decreased elasticity of the sclerotic bone. If an intraoperative fracture occurs, fixation with locking screws from the baseplate can be attempted, while small marginal fractures can be ignored [11]. However, if such fixation is not possible, fragment-specific fixation using a screw or wire is the second choice. In catastrophic glenoid failure, bone grafting and fixation of the glenoid implant should be discussed. These processes can be performed in one or two stages (prepare the bone graft and glenoid bone stock and re-implant the glenoid component). However, negative outcomes have been reported in cases of glenoid fracture [6].
Our first case of periprosthetic fracture was intraoperative glenoid fracture during trial reduction. This was the first case of RSA in our department for 12 years. Therefore, we had to convert to hemiarthroplasty, since our experience of RSA was limited (Fig. 4).
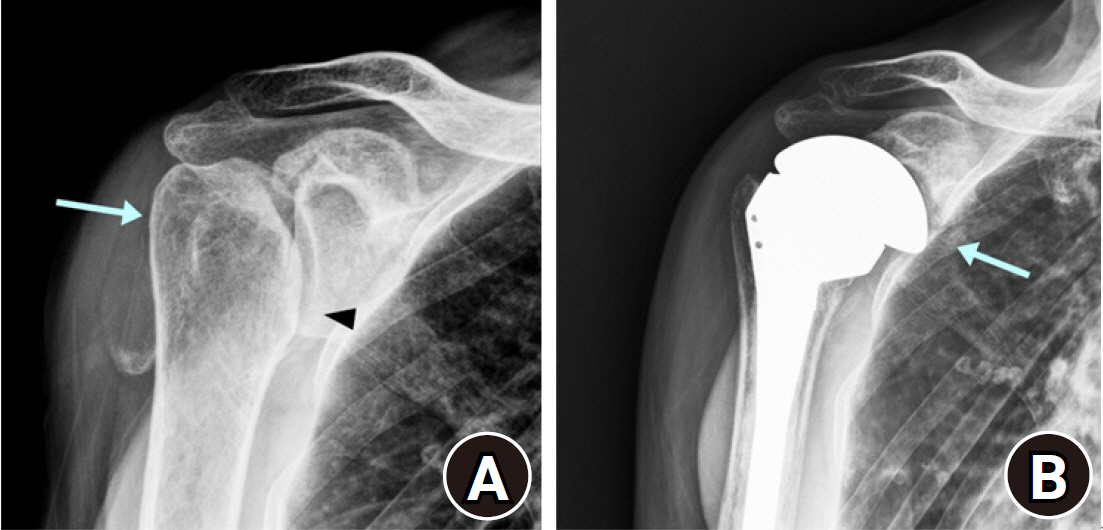
Intraoperative glenoid fracture during reverse shoulder arthroplasty. (A) Preoperative humeral head deformity (arrow) with glenohumeral joint space narrowing was observed (arrowhead). (B) Glenoid fracture occurred during the reduction procedure, and surgery was converted to hemiarthroplasty (arrow).
ACROMION/SCAPULAR SPINE FRACTURE
Acromial or scapular spine fracture after RSA is a rare complication, with incidence rates ranging from 0.9% to 10% [11,15,51]. The acromion and spine of the scapula are the origins of the deltoid muscle. The mean arm length increases by 2.5 cm with distalization of the humerus, and the center of rotation is medialized after RSA, increasing the tension in the deltoid muscle [52]. In addition, during arm elevation after RSA, the deltoid muscle acts as an elevator, and the load on the acromion is increased. Increased tension and load can cause stress fracture of the acromion or scapular spine.
Initially, patients experience lateral shoulder pain with decreased shoulder function [11,12]. Moreover, the patients are not able to elevate their arm due to loss of tension in the deltoid muscle [11]. The fracture can be treated with conservative treatment, such as shoulder immobilization, or surgical treatment, but this is debated. Levy et al. [53] reported 18 cases of conservative treatment of acromion/spine fracture, and the patients showed decreased shoulder function, while Hattrup [55] reported good results after conservative treatment of acromion/spine fracture. In scapular spine fracture, Crosby et al. [51] reported high nonunion rate after conservative treatment and recommend surgical treatment with tension band wiring and buttress plate.
Osteoporosis and acromial erosion before surgery are risk factors for acromion or scapular spine fractures [11,12]. Excessive distalization of the humerus and medialization of the center of rotation result in high tension in the deltoid muscle. Hence, surgeons should not position the baseplate excessively inferiorly to avoid humeral overdistalization in patients with osteoporosis or a thin acromion. In addition, use of a lateralized-type prosthesis rather than a Grammont-type prosthesis is recommended to prevent overtension of the deltoid muscle. In addition, malpositioning of the superior or posterior baseplate screws is associated with scapular spine stress fracture [11]. Pointing the upper screw of the baseplate toward the coracoid base could prevent scapular spine fractures. During postoperative outpatient visits, surgeons should assess the serial changes in acromion tilt on plain radiography and should cautiously investigate percussion tenderness over the acromion.
We report two cases of an acromial stress fracture after RSA. The first patient recovered with conservative treatment, while the second patient required arthroscopic rotator cuff repair for a medium-sized rotator cuff tear with acromioplasty and did well for 5 years. The patient then complained of increasing right shoulder pain; hence, we performed RSA with a lateralized glenoid design. Before surgery, she had osteopenia (T-score, –0.6) and her acromion was 4.8 mm thick. After surgery, the humerus was distalized by 2.4 cm. Four months after surgery, she developed new-onset lateral shoulder pain, and plain radiograph showed inferior tilt of the lateral acromion. Conservative management was performed for 2 years. Although shoulder function improved gradually, she showed a low degree of shoulder elevation (active forward elevation, 80°) and low functional scores (Fig. 5).
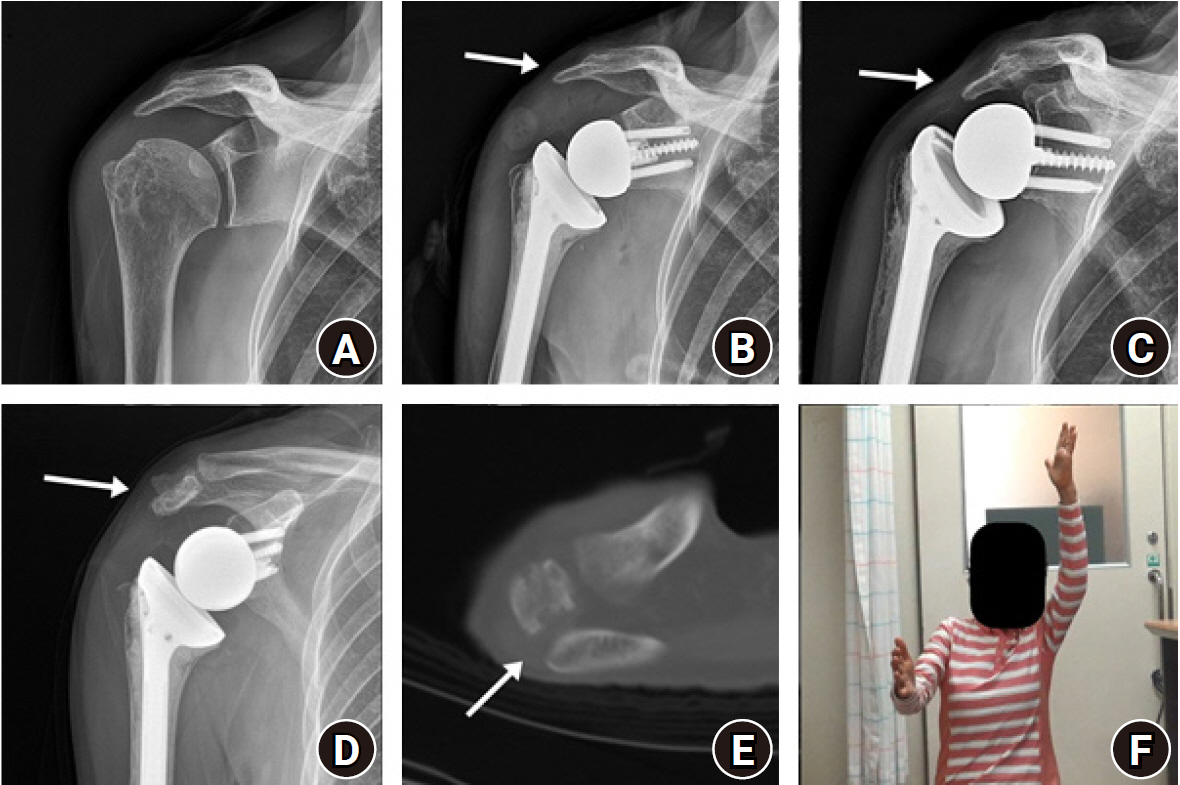
Acromion fracture after reverse shoulder arthroplasty (RSA). (A) Initial anteroposterior (AP) radiograph and (B) early postoperative AP radiograph showed an intact acromion (arrow). (C) Acromial inferior tilt (arrow) was observed at 4 months after RSA. Two years after RSA, (D) inferior tilt of acromion (arrow) and (E) non-union of acromion (arrow) were observed on computed tomography. (F) The patient had decreased right shoulder elevation at 2 years after surgery (photograph used with permission for study purpose).
SCAPULAR NOTCHING
Scapular notching is a unique complication of RSA resulting from changes in the glenohumeral anatomical structure [55]. It is usually observed 6 months postoperatively on plain radiography, and the reported incidence of scapular notching ranges from 4.6% to 96% [6,8,11,56,57]. Scapular notching is the most commonly observed complication; hence, Zumstein et al. [6] classified this as a postoperative problem rather than a complication. Notching refers to mechanical impingement of the humeral component at the scapular neck during extension and external rotation at the side [57]. The main position of notching is the posteroinferior aspect of the scapular neck, but it can occur at the anteroinferior aspect of the neck [56].
The occurrence of notching depends on multiple factors, such as implant design and position, patient anatomy, and range of motion [11,57]. The Grammont-type implant has a high tendency for scapular notching because of the large neck shaft angle of the humeral stem [56]. In a study by Kolmodin et al. [56], scapular notching was observed in 59% of cases with Grammont-type prostheses. A decreased humeral neck shaft angle tends to protect against scapular notching [58]. In addition, inferior glenosphere placement, inferior tilt, and lateralization of the center of rotation are thought to decrease the risk of notching [57].
Decreased scapular neck length (SNL) leads to an increased rate of scapular notching [59]. SNL is determined innately; however, it can be shortened by wear of the glenohumeral joint by cuff tear arthropathy or degenerative/inflammatory arthritis [57]. In cases of short SNL, glenoid lateralization using an eccentric glenosphere or glenoid augmentation should be considered [57,59].
The clinical course of scapular notching is debated [60]. Many studies have reported that patients without scapular notching showed better range of motion and functional outcomes than patients with scapular notching [57,60]. Mollon et al. [58] observed scapular notching with a single implant (medial glenoid/lateral humerus design) in 10% of 476 cases and found that patients with scapular notching showed poor functional scores, low degree of shoulder elevation, and reduced muscle strength [58].
In addition, patients with scapular notching showed significantly higher complication rates and tended to have significantly higher rates of humeral radiolucent lines than patients without scapular notching [58]. Notching grades 1 and 2 are thought to be caused by mechanical friction, while grades 3 and 4 are considered to be biological responses to polyethylene particles resulting from humeral or glenoid osteolysis [57]. Notching induces wear of the polyethylene, resulting in osteolysis of the glenohumeral joint [58].
There are a few methods to prevent scapular notching. Lateralization of the glenoid component is one method. When performing RSA in patients with SNL less than 9.0 mm, glenoid augmentation should be considered or an implant with increased lateral offset can be used [57]. In addition, use of an eccentric glenosphere is helpful [61]. Inferior overhang of the glenosphere of 3–4 mm prevents scapular notching [56,57]. Inferior tilt of the glenosphere by 15°–20° also prevents notching [61]; use of lateralized humeral prostheses increases postoperative external rotation and decreases the risk of scapular notching [13]. According to Ferrier et al. [62], the best clinical results and lowest incidence of scapular notching were found after lowering the humerus by more than 24 mm.
NEUROLOGIC COMPLICATIONS
Most minor neurologic complications after RSA cannot be detected. The previously reported incidence of neurologic complications was 1%–4% after RSA [63]. Neurologic complications can occur during or after surgery. The most commonly injured nerves after RSA are the axillary nerve and brachial plexus [7,64]. In addition, suprascapular nerve and recurrent laryngeal/hypoglossal nerve injuries have been reported [65,66]. These injuries are generally reversible during the first 3 months after surgery, but some do not heal for long periods, resulting in neurologic deficits [11].
The axillary nerve originates from the posterior cord of the brachial plexus, runs anterior to the subscapularis, lies under the inferior capsule and glenoid rim, and runs through the quadrilateral space. Anatomically, the axillary nerve passes 3.2–12.4 mm below the inferior glenoid rim [65,67]. The nerve then divides into the anterior and posterior branches, and the anterior branch wraps the inner surface of the deltoid muscle, which is 5–7 cm distal to the lateral acromial border, and innervates the deltoid muscle [67-69]. Injury to the axillary nerve causes deltoid dysfunction, resulting in difficulty in elevating the shoulder. In addition, decreased anterior-to-posterior deltoid tension can cause instability [68]. Additionally, gross wasting of the shoulder, persistent shoulder pain, and impaired rehabilitation can be observed [68].
The most common site of injury to the axillary nerve during surgery is the inferior glenoid rim [65]. During glenoid preparation, iatrogenic injury can occur due to prolonged retraction and wide exposure with electrocautery [65]. Deep and sharp retractors such as the Hormann retractor should be used cautiously, and careful periosteal detachment of the capsulolabral tissue for glenoid preparation is necessary to prevent iatrogenic axillary nerve injury. However, axillary nerve injury can be difficult to detect immediately after surgery since the operated shoulder usually is immobilized. In addition, Lädermann et al. [68] reported axillary nerve injury at the posterior humeral metaphyseal level. They found that the axillary nerve to the deltoid muscle is close to the posterior humeral component; therefore, caution should be exercised when cutting the humeral neck and reaming to avoid damage to the posterior humeral cortex.
Injury to the brachial plexus can be caused by humerus positioning during surgery. During the deltopectoral approach, excessive humeral hyperextension, external rotation, and anterior translation of the humeral head can damage the brachial plexus [38]. Van Hoof et al. [70] reported a 15.3%–19.3% increase in strain at the median nerve root after surgery using a three-dimensional computer model. In addition, Lynch et al. [71] observed nerve injury during shoulder joint replacement surgery in 18 of 417 patients, and most injuries were neurapraxias from stretching injury due to positioning. Excessive humeral distalization also can cause traction injury of the brachial plexus during or after surgery [11,71].
We observed one case of brachial plexus palsy among 438 RSA cases; the patient recovered only partially after 3 years of follow-up. The patient was a 77-year-old woman who showed reduction in strength for shoulder elevation, wrist drop, and reduction in grasp power just after the surgery. Two years after surgery, a nerve conduction study showed a brachial plexus lesion (Fig. 6). One possible cause of injury was excessive positioning such as hyperextension, external rotation, and anterior translation during the humeral procedure. Since then, we have taken caution to avoid such situations. We have since performed RSA in 236 patients, and none experienced brachial plexus injury.
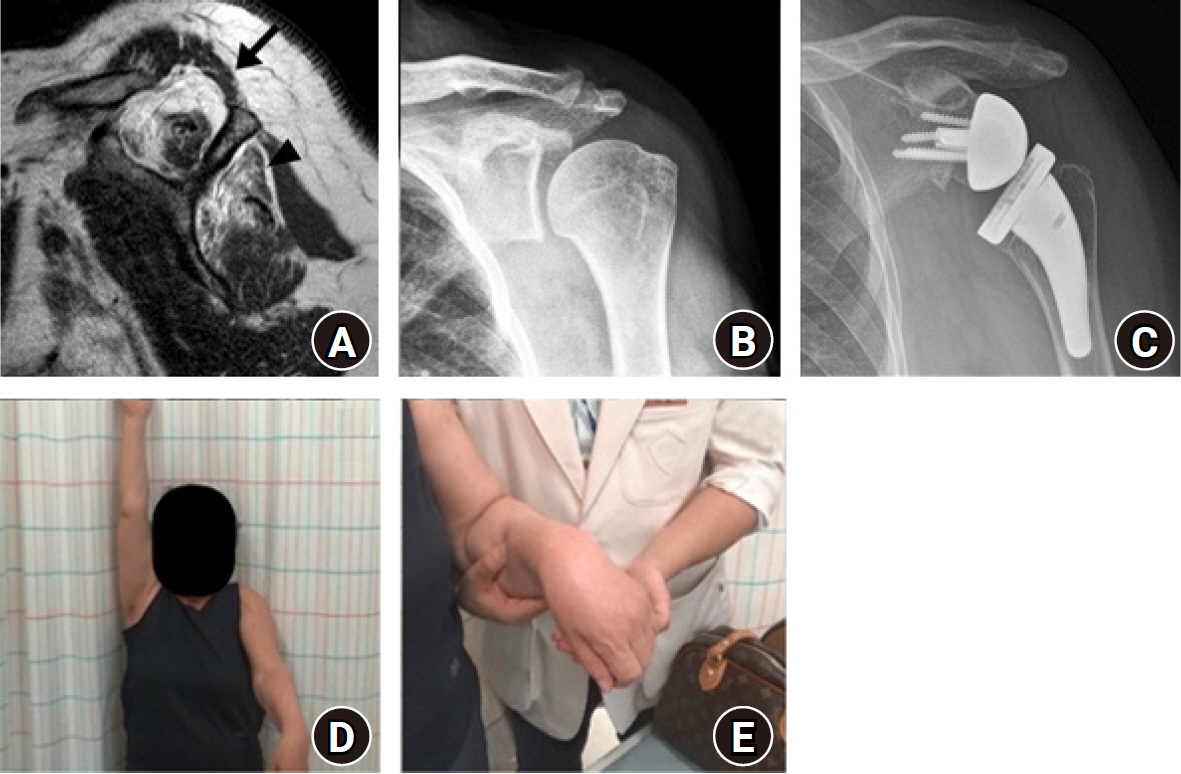
Brachial plexus injury after reverse shoulder arthroplasty. (A) Preoperative scapular Y-view of magnetic resonance imaging showed greater than 50% muscle atrophy in the supraspinatus (arrow) and infraspinatus (arrowhead). Preoperative (B) and postoperative (C) anteroposterior radiographs of the shoulder. (D) Decreased left shoulder elevation and (E) wrist drop sign were observed during follow-up, indicating brachial plexus injury (photograph used with permission for study purpose).
Malpositioning of screws can be associated with suprascapular nerve injury during glenoid fixation. Extraosseous placement of superior and posterior screws can damage the suprascapular nerve at the scapular notch or spinoglenoid notch [11]. In addition, excessive head tilting during surgery could cause recurrent laryngeal nerve injury or hypoglossal nerve injury, resulting in Tapia’s syndrome [66].
CONCLUSION
Given the increase in number of patients undergoing RSA, the number of patients experiencing complications is increasing. Although it is difficult to manage complex complications after RSA, such as dislocation, infection, periprosthetic fracture, and aseptic loosening of the prosthesis, complications can be corrected by determining the underlying reasons and planning the treatment strategy accordingly. Importantly, surgeons should be cautious in addressing the pre- and intraoperative factors of complications to reduce the incidence of complications such as dislocation, scapular notching, and neurologic complications.
Notes
Financial support
None.
Conflict of interest
None.
