An objective assessment of the impact of tendon retraction on sleep efficiency in patients with full-thickness rotator cuff tears: a prospective cohort study
Article information
Abstract
Background
Sleep quality, quantity, and efficiency have all been demonstrated to be adversely affected by rotator cuff pathology. Previous measures of assessing the impact of rotator cuff pathology on sleep have been largely subjective in nature. The aim of the present study was to use an objective measure of sleep quality and to compare these findings to the patients’ Patte stage.
Methods
Patients with full-thickness rotator cuff tears at a single institution were prospectively enrolled between 2018 and 2020. Waist-worn accelerometers were provided for the patients to use each night for 14 days. Sleep efficiency was calculated using the ratio of the time spent sleeping to the total amount of time that was spent in bed. Retraction of the rotator cuff tear was classified using the Patte staging system.
Results
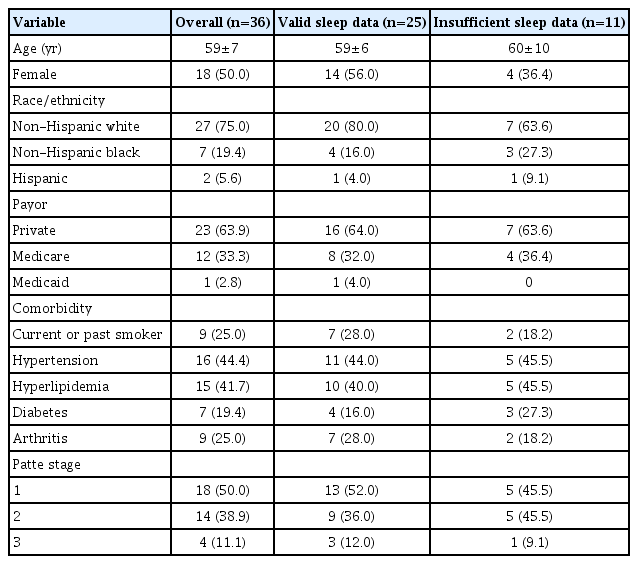
This study included 36 patients: 18 with Patte stage 1 disease, 14 with Patte stage 2 disease, and 4 patients with Patte stage 3 disease. During the study, 25 participants wore the monitor on multiple nights, and ultimately their data was used for the analysis. No difference in the median sleep efficiency was appreciated amongst these groups (P>0.1), with each cohort of patients demonstrating a generally high sleep efficiency.
Conclusions
The severity of retraction of the rotator cuff tear did not appear to correlate with changes in sleep efficiency for patients (P>0.1). These findings can better inform providers on how to counsel their patients who present with complaints of poor sleep in the setting of full-thickness rotator cuff tears.
Level of evidence
Level II.
INTRODUCTION
Rotator cuff pathology is a condition often seen in the orthopedic clinic and is the most common cause of shoulder-related disability in patients [1]. Up to 54% of patients 60 years of age or older have imaging findings that are suggestive of a partial or complete rotator cuff tear [2]. While not all of these patients are symptomatic and therefore require intervention, the fact remains that nearly 250,000 repairs are completed annually in the United States alone [2]. Given the prevalence of rotator cuff disease in the population, it becomes paramount to determine how best to counsel patients regarding activities of daily living.
One component of life that is affected by a rotator cuff tear is sleep. Classically, symptomatic rotator cuff tears have been associated with poor sleep quantity and quality [3]. In fact, up to 89% of patients with symptomatic rotator cuff tears report abnormal sleep [4-6] compared to a reported 35% prevalence of sleep disturbances in the general population [6-8]. In some cases, issues with sleep can be the driving force that leads patients to seek care rather than shoulder pain or limited shoulder function [9]. Poor sleep has been associated with many downstream adverse medical conditions, including depression, heart disease, stroke, diabetes mellitus, obesity, and hypertension [7,8,10]; such consequences help to underscore the importance of identifying and addressing underlying causes of poor sleep.
The correlation between sleep disturbances and rotator cuff injuries has been investigated in recent years. Many studies have used self-report questionnaires, such as the Pittsburg Sleep Quality Index (PSQI) and the Epworth Sleepiness Scale, to assess sleep quality in patients with rotator cuff tears. These are validated [8,11] yet subjective measures, but studies that have employed these scoring systems have yet to demonstrate a consistent association in this patient population [10,12-15].
Multiple studies have attempted to investigate the association between specific characteristics of rotator cuff tears and sleep quality. Gumina et al. [14] evaluated subjective sleep data and categorized their patients based on the intraoperative determination of their rotator cuff tear size as follows: small, large, or massive tears. They concluded that patients with smaller tears had worse sleep quality compared to those with larger tears, both regarding the time required to fall asleep and also the level of sleep disruption. Reyes et al. [15] utilized magnetic resonance imaging (MRI) findings to identify an association between PSQI questionnaire responses and rotator cuff tear size, tendon retraction, Goutallier grade, muscle atrophy, number of tendons involved, and presence of a high riding humeral head. These qualities of the rotator cuff tear were not shown to be correlated with patients’ subjective interpretations of sleep.
Per our literature review, only one study thus far has sought to objectively assess the impact of rotator cuff tears on sleep. Ansok et al. [12] used a monitor worn on the wrist to utilize actigraphy, a validated model for the purposes of researching sleep quality, to evaluate patients with rotator cuff tears. They concluded that patients with rotator cuff tears demonstrated a statistically significant difference compared to historical cohorts when evaluating their mean total sleep time, sleep onset latency, sleep efficiency, and time until arousal after sleep onset. Additionally, it was noted that subjective and objective measures of sleep did not appear to correlate [12].
The aim of the present study was to use an objective measure of sleep quality and to compare these findings to the patients’ Patte stage. An Actical accelerometer worn on the waist was used to evaluate patients’ sleep efficiency, which is the ratio of total time asleep compared to the total time in bed [16]. By ascertaining this relationship, providers will have additional data they may use to counsel patients and provide guidance on the management of their patients’ symptomatic rotator cuff tears. Our hypothesis was that a higher Patte stage would be associated with a poorer sleep efficiency.
METHODS
This study was approved by Institutional Review Board of Loyola University Medical Center (No. LU #211042), and informed consent from patients was obtained.
Patient Characteristics
A prospective cohort study was carried out with patients seen in the clinics of two Loyola University Medical Center orthopedic surgeons (NGG and DHS) who were determined to meet the inclusion and exclusion criteria through a chart review and verbal interview. The inclusion criteria for this study were as follows: patients between the ages of 30 and 70 with a diagnosis of a full-thickness rotator cuff tear on MRI or ultrasound, and those who were symptomatic and seeking treatment. Those with conditions such as glenohumeral osteoarthritis and adhesive capsulitis were identified based on their clinical and radiographic findings and were not included in our cohort; these patients were excluded to minimize the chance that alternative pathologies might be contributing to the patient’s sleep difficulties. Full-thickness rotator cuff tears were diagnosed when the synovial fluid appeared contiguous with the subacromial fluid on single or adjacent MRI slices. Patients who had a history of a prior shoulder surgery, previously documented sleep disorders, used over-the-counter or prescription sleep medication, were night shift workers, were new mothers/fathers (within 6 months of childbirth), were pregnant or nursing females, and were habitual late-night caffeine drinkers (within three hours of their usual bedtime) were excluded from the study.
Study variables included sleep efficiency as measured by a waist-worn Actical accelerometer and tendon retraction as classified by Patte stage. The Actical accelerometer was specifically selected because it is a validated product that is readily available to our department and was previously used by the authors to investigate sleep efficiency.
Assessment of Sleep Efficiency
The Actical accelerometer, which is manufactured by Philips Respironics, is an omnidirectional sensor that records acceleration values at specified time points [16,17]. The participants were given verbal and written instructions on how to wear and use the accelerometer. The patients were asked to wear the activity monitor around their waist on the right side of their body at all times for 14 days except during showering, bathing, or swimming. The 14-day time period was chosen in an effort to improve compliance and was also based on recommendations of those familiar with the product and software. The participants were asked to press an indicator button on the device before going to sleep and then again after waking up. For each participant, the nightly sleep efficiency was calculated as the percentage of nighttime minutes worn with activity detected based on a moving average (current minute ±1 minute) as defined by Borghese et al. [16].
Imaging Assessment
The Patte stage is determined using the classification scheme of rotator cuff tears that was proposed by Didier Patte in 1989. This method uses MRI images to describe the tendon retraction seen in rotator cuff tears and can help predict the reparability of these rotator cuff tears [18]. This scheme examines three characteristics: the extent of the tear, the topography of the tear in the sagittal plane, and the topography of the tear in the coronal plane. The topography of the tear in the coronal plane is staged based on the location of the proximal stump relative to the bony landmarks of the shoulder (termed the “Patte stage” in this publication). Stage 1 represents a proximal stump close to the bony insertion; stage 2 indicates a proximal stump at the level of the humeral head; while stage 3 represents a proximal stump at the level of the glenoid [19]. Patte stage was determined via an MRI review of three consecutive slices by two faculty members and one senior resident in the Department of Orthopedics; 3/3 or 2/3 interobserver agreement between the researchers was used to determine the final Patte stage.
Statistical Analysis
Sleep efficiency across 14 nights was analyzed using the days where patients recorded at least 8 hours of total wear time. The association between age and sleep efficiency was described using the Spearman’s rho. The distribution of sleep efficiency was compared across demographic subgroups using Wilcoxon rank-sum tests. Statistical analyses were performed using SAS 9.4 (SAS Institute).
RESULTS
Demographics
Between August 2018 and June 2020, 36 patients were enrolled in this study. The mean age was 59±7 years; 18 of the participants were female, and the majority was Caucasian (n=27, 75.0%) and had private insurance (n=23, 63.9%). The most common comorbidities were hypertension (n=16, 44.4%) and hyperlipidemia (n=15, 41.7%). One quarter were current or former smokers (n=9). Half had Patte stage 1 retraction, while 14 (39.9%) had Patte stage 2 and 4 (11.1%) had Patte stage 3 (Table 1) retraction.
Sleep Efficiency
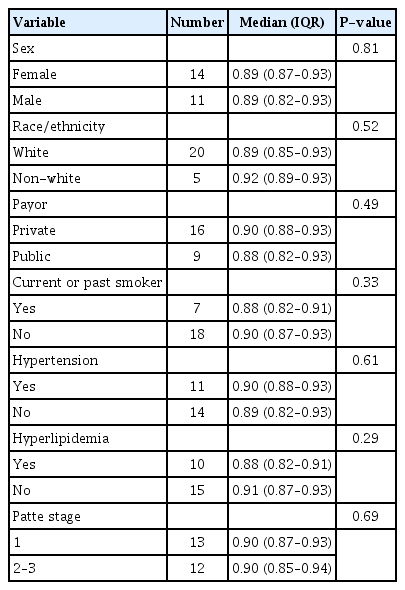
In all, 25 patients wore the accelerometer on multiple days and contributed to the sleep efficiency analysis. The average sleep efficiency was high across all demographic subgroups with a median ≥88% for each subgroup (Table 2). A small inverse relationship between age and sleep efficiency was also observed (rho=–0.11), although this was not statistically significant. No statistically significant differences in sleep efficiency amongst the subgroups of Patte stages were appreciated upon an analysis of the data. Of note, the patients with Patte stages 2 and 3 were combined for the analysis due to the limited number of patients with a classification of a Patte stage 3 tendon retraction. The 11 patients who were not included in the final analysis did not demonstrate consistent use of the accelerometer, which was defined as a minimum of 8 hours of wear time nightly across 14 days.
DISCUSSION
In this prospective cohort comparing objective sleep efficiency data with rotator cuff tendon retraction, we found no significant difference in the quality of sleep between patients with Patte stages 1, 2, and 3. These findings are consistent with those by Reyes et al. [15], who reported that MRI findings did not correlate with subjectively reported poor sleep efficiency. Additionally, it was noted that the mean sleep efficiency amongst the included participants was 89% in the present study, which was a value mirrored by the mean sleep efficiency of 89% in the study that was used to validate the Actical accelerometer [16].
Evidence regarding the correlation of subjective sleep questionnaires such as the PSQI and rotator cuff tears is conflicting. Some studies have shown no correlation between the presence or the absence of primary rotator cuff tears and subjective assessments of sleep [10,14]. Other data demonstrate that repair of the rotator cuff subjectively improves sleep quality to close to that of the general population [6,7,9,12,13,20]. Additionally, rupture of repaired rotator cuffs has been shown to subjectively decrease sleep quality compared to that of patients without re-rupture [7]. Given the paucity of consistent evidence on this topic, studies that utilize objective measures of sleep have been undertaken. Ansok et al. [12] demonstrated not only an association between the presence of rotator cuff pathology and diminished sleep quantity and efficiency but also a poor correlation between the PSQI and objective data, as patients were found to overestimate both their sleep quality and quantity.
The impact of poor sleep quantity and quality cannot be overstated. Insomnia has been shown across the literature to be detrimental to emotional, cognitive, and physical health [10,21]. Emotionally, sleep disturbances are associated with heightened negative affect towards unpleasant events and a dulled response to neutral events in otherwise healthy patients [22]. Insomnia is associated with absenteeism, loss of productivity, debilitated memory, and impaired executive functioning, all of which can have a negative impact on workplace efficiency and decision-making [23]. In addition to the medical consequences of poor sleep, such as an increased risk of depression, obesity, and heart disease, insomnia has been associated with decreased human growth hormone production, which in turn disrupts the process of tissue healing [9,20].
Sleep disturbances in patients with rotator cuff tears can be multifactorial [5,10,13]. One independent contributor to poor sleep would be the use of narcotic pain medication [7,9]. The mental health of the patient has also been shown to play a role in how patients manage living with a rotator cuff tear. One study by Wylie et al. [24] looked for an association between the patient’s mental health as defined using a validated screening tool, the Short Form-36 Mental Component Summary (SF-36 MCS), and objective measurements of rotator cuff tear size with patient-reported outcomes. The SF-36 MCS was found to have a stronger association with the visual analog scale, the Simple Shoulder Test, and the American Shoulder and Elbow Surgeons score than the correlation between MRI findings of tear severity and patient-reported outcome measures. Poor mental health and conditions including depression, anxiety, and substance use disorders are often closely associated with disordered sleeping, which can thus be an independent factor when evaluating sleep efficacy in this patient population [8].
The relationship between nocturnal pain and rotator cuff tears has yet to be completely understood. The prevailing idea is that pain at night is due to an increase in the release of pro-inflammatory cytokines [21]. These pro-inflammatory cytokines increase the expression of melatonin receptors in the injured shoulder, which act to increase proinflammatory cytokine release and pain receptor expression with a resultant positive feedback loop [25]. An additional source of nocturnal pain is increased perfusion to the torn rotator cuff tissue. A study by Terabayashi et al. [26] found a relative increase in blood flow through the anterior humeral circumflex artery in injured shoulders compared to non-injured shoulders—a phenomenon that was not demonstrated in healthy volunteers or in patients with rotator cuff tears without nighttime symptoms.
This study is not without limitations. One would be the application of the algorithm that was used to calculate sleep efficiency; this study included adults from 30 to 70 years old, while the original validation study by Borghese et al. [16] evaluated sleep efficiency in 10–13-year-old children. The small sample size of this study reduces its power and thus increases the chance of missing a significant difference between the subgroups. Additionally, this study did not include a control group without rotator cuff tears. Regarding the study design, there are biases that may have influenced the results as follows: selection bias, as patients who chose to enroll may have a generally poorer sleep quality, and the Hawthorne effect, as patients knew they were being evaluated, which could have affected their sleep. Lastly, while the Patte stage was used as a surrogate of rotator cuff tear size, this classification may not capture certain tears that are larger in the sagittal plane or that are not accompanied by retraction. Future studies can build off of these data and potentially consider calculating the area of the tear as an alternative method of classification.
CONCLUSIONS
This study found that there was no association between the MRI findings of rotator cuff tear severity and a patient’s objective sleep quality. While further research is warranted with a larger sample size, these data provide preliminary information that for patients with full-thickness rotator cuff tears, there is no difference in sleep efficiency related to tear size. While the Patte stage does indicate the reparability of the rotator cuff, it does not present an opportunity to correlate the clinical and imaging findings in patients with complaints of nocturnal shoulder pain.
Notes
Author contributions
Conceptualization: RB, AS.Data curation: WD, RB, AS. Formal analysis: LRD, CJ. Investigation: RB, AS. Methodology: RB, DHS, NGG. Supervision: DHS, NGG. Writing – original draft: AEM, WD. Writing – review & editing: AEM.
Conflict of interest
None.
Funding
None.
Data availability
Contact the corresponding author for data availability.
Acknowledgments
These data were presented as a poster entitled “Objective sleep assessment in patients with rotator cuff disease: a prospective study.” This presentation was given at the Mid-America Orthopedic Association on April 8, 2022.
We thank Hassan Farooq (Department of Orthopedic Surgery and Rehabilitation, Loyola University Health System) for his contributions for the creation of tables and figures, the initial statistical analysis, and abstract preparation.