Arthroscopic transosseous anchorless rotator cuff repair reduces bone defects related to peri-implant cyst formation: a comparison with conventional suture anchors using propensity score matching
Article information
Abstract
Background
The transosseous anchorless repair (ToR) technique was recently introduced to avoid suture anchor-related problems. While favorable outcomes of the ToR technique have been reported, no previous studies on peri-implant cyst formation with the ToR technique exist. Therefore, this study compared the clinical outcomes and prevalence of peri-implant cyst formation between the ToR technique and the conventional transosseous equivalent technique using suture anchors (SA).
Methods
Cases with arthroscopic rotator cuff repair (ARCR) between 2016 and 2018 treated with the double-row suture bridge technique were retrospectively reviewed. Patients were divided into ToR and SA groups. To compare clinical outcomes, 19 ToR and 57 SA cases without intraoperative implant failure were selected using propensity score matching (PSM). While intraoperative implant failure rate was analyzed before PSM, retear rate, peri-implant cyst formation rate, and functional outcomes were compared after PSM.
Results
The intraoperative implant failure rate (ToR, 8% vs. SA, 15.3%) and retear rate (ToR, 5.3% vs. SA, 19.3%) did not differ between the two groups (all P>0.05). However, peri-implant cysts were not observed in the ToR group, while they were observed in 16.7% of the SA group (P=0.008). Postoperative functional outcomes were not significantly different between the two groups (all P>0.05).
Conclusions
The ToR technique produced comparable clinical outcomes to conventional techniques. Considering the prospect of potential additional surgeries, the absence of peri-implant cyst formation might be an advantage of ToR. Furthermore, ToR might reduce the medical costs related to suture anchors and, thereby, could be a useful option for ARCR.
Level of evidence
III.
INTRODUCTION
Arthroscopic rotator cuff repair (ARCR) using suture anchors has gradually replaced the open classical transosseous repair technique. The first generation of suture anchor designs consisted of nonabsorbable and metallic materials. While metallic suture anchors could offer firm initial fixation, several complications including migration, loosening, entrapment of metal anchors within the joint cavity, and hindrance of diagnostic imaging due to artifacts were associated with their use [1,2]. To overcome these limitations, new types of suture anchors have been continuously developed, progressing toward smaller diameters with more suture threads to reduce bone defects. However, there are still unresolved problems associated with suture anchors, including increased bone defects due to peri-implant cyst formation, pull-out, and economic costs [3-7].
Therefore, several transosseous tunneling systems have been introduced to repair torn rotator cuff tendons without suture anchors to avoid suture anchor-related problems [8-10]. These systems mimic the open classical transosseous repair technique without suture anchors and have presented favorable outcomes [8-10]. However, there were several concerns about the initial stability of transosseous anchorless repair (ToR). In biomechanical tests, the suture anchor presented stronger fixation than the classical open transosseous repair [8,11], with the most common failure mechanism of ToR being cutting through the bone with the suture material [11].
The mechanism of ToR depends on the tension of the knotted suture materials that penetrate the tuberosity of the proximal humerus. Therefore, local bone mineral density (BMD) of the proximal humerus might affect the failure of ToR [12]. When estimating local proximal humeral BMD, several factors that could affect it including chronological age, sex, hand dominance of the affected shoulder, and size of the torn rotator cuff should be considered [13-18].
Furthermore, as ToR is not fixed at a single point as a suture anchor, micromotion could be provoked during the range of motion (ROM), which has been estimated as one of the causes of peri-implant cyst formation after ARCR using suture anchors [19]. However, no previous studies exist on peri-implant cyst formation with ToR using transosseous tunneling systems.
This study sought to compare clinical outcomes, retear rates, and occurrence of peri-implant cyst formation between the ToR technique and the conventional transosseous equivalent technique using suture anchors (SA). We hypothesized that the ToR technique and the conventional transosseous equivalent technique utilizing suture anchors would yield comparable clinical outcomes and retear rates. Additionally, the incidence of peri-implant cyst formation could potentially be reduced in the ToR group.
METHODS
The protocol of this study, including the waiver of patient informed consent, was approved by Institutional Review Board of Seoul National University Bundang Hospital (No. B-2211-792-103).
All primary ARCR cases performed by a single surgeon (JHO) at the institution of the senior author using the double-row suture bridge technique (DRSB) to treat medium to large-sized rotator cuff tears between January 2016 and January 2018 were retrospectively reviewed. The authors included primary ARCR cases to treat supraspinatus and/or infraspinatus tears using DRSB with the ToR technique or conventional SA that had been followed for at least 2 years.
To reduce heterogeneity, cases with concurrent subscapularis repair were excluded. In this study, intraoperative implant failure was defined as bone cut-through by suture material in the ToR group and pulling out of the suture anchor in the SA group. Cases with intraoperative implant failure were only considered in the analysis of intraoperative failure rate and were excluded from the assessment of functional and radiological outcomes. To minimize bias originating from confounders, propensity score matching (PSM) was performed at a ratio of 1:3 using sex, age at time of surgery, hand dominance of the operation side (dominant or non-dominant arm), tear size, and systemic BMD.
A retrospective assessment of medical records, including the physician's admission and progress notes, operation records, anesthesia records, functional score results, and radiographs, was performed. To evaluate the baseline characteristics and gather the variables for PSM, demographic factors, including age at operation, sex, operation side (dominant or non-dominant arm), and systemic BMD measured using dual-energy X-ray absorptiometry (DXA) were corrected. To compare the medical costs of the surgical techniques, the number of suture anchors used and operation time were considered.
Functional outcomes were evaluated with the visual analog scale of pain, active ROM including forward flexion, external rotation of the arm at the side, and internal rotation of the arm at the back, the American Shoulder and Elbow Surgeons standardized shoulder assessment form, simple shoulder test, and the abbreviated version of disability of the arm, shoulder, and hand. All functional outcomes were evaluated preoperatively and at the final follow-up visit. Forward flexion and external rotation were measured in the neutral position and with the arm in the side position, respectively, using a goniometer. Internal rotation was measured at the height of the spinous process, which was accessible with the ipsilateral thumb [20].
To compare the radiological outcomes, fatty degeneration of each rotator cuff muscle, presence of retear, and peri-implant cyst formation were evaluated. magnetic resonance imaging (MRI) was performed during the preoperative work-up and at the postoperative 1-year follow-up. Ultrasonography was performed at 3 and 6 months postoperatively and every annual follow-up visit from 2 years postoperatively.
Fatty degeneration was evaluated using Goutalier-Fuchs grade in the scapular Y-view on the sagittal plane of preoperative MRI [21,22]. The presence of retears in the repaired rotator cuff tendon was evaluated using MRI and ultrasonography. Retears on MRI were defined as Sugaya classification type IV or V [23]. The criteria for retear during the ultrasonography were non-visualization or focal defects (hypoechoic or mixed hypoechoic and hyperechoic lesions) of the repaired tendon with consecutive loss of the normal arc of the subdeltoid bursa [24,25]. The degree of fatty degeneration and the presence of retear were evaluated with a formal reading by a musculoskeletal radiologist blinded to this study.
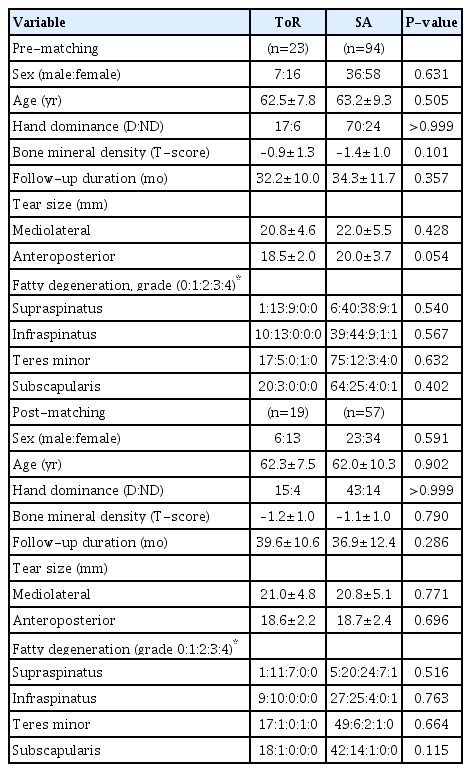
Peri-implant cyst formation was defined as a fluid signal around the suture material in the ToR group or the medial suture anchor in the SA group on T2-weighted MRI at postoperative 1-year follow-up (Fig. 1) [5-7]. Since the shapes of the suture material and anchor were quite different, the classification of the peri-implant cyst was simplified to a binary value (presence or absence of peri-implant cyst) to decrease the heterogeneity originating from the morphologic difference of implants. In addition, two orthopedic surgeons (HJJ and JSL), evaluated peri-implant cyst formation independently to calculate the intra- and inter-observer reliability, with at least 2 weeks intervals between measurements.

T2-weighted coronal magnetic resonance imaging scans at 1 year postoperative. (A) Transosseous anchorless repair. The transosseous tunnel (white arrowheads) was observed. There was no fluid collection around the transosseous tunnel. (B) Transosseous equivalent repair using conventional suture anchors. There was obvious fluid collection around the medial row suture anchor (yellow arrowhead).
Surgical Technique and Rehabilitation Protocol
All surgical procedures were performed in the lateral decubitus position using the Spider Limb Positioning System (Smith & Nephew) under general anesthesia. Presence of the subscapularis tear was evaluated in the intra-articular space. The indications for subscapularis repair were a full-thickness tear or a partial-thickness tear involving more than half of the total thickness of the subscapularis tendon. Tear size of the supraspinatus and/or infraspinatus was measured in both mediolateral (retraction) and anteroposterior directions using a probe in the subacromial space with a 70° arthroscope. Only DRSB was conducted in cases with a robust tendon pulled through to the tip of the greater tuberosity without resistance after sufficient subacromial decompression and release of adjacent soft tissues around the torn rotator cuff [26].
Conventional DRSB repair (Fig. 2) was conducted with four suture anchors. Two medial suture anchors were inserted just lateral to the articular surface. The same pair of suture threads from the medial anchors penetrated the torn tendons at intervals of 4 mm and were tied to press the torn tendon down to the medial portion of the greater tuberosity. Sequentially, two lateral anchors were inserted into the lateral portion of the greater tuberosity, where the surface area between the torn tendon and the greater tuberosity was maximized.
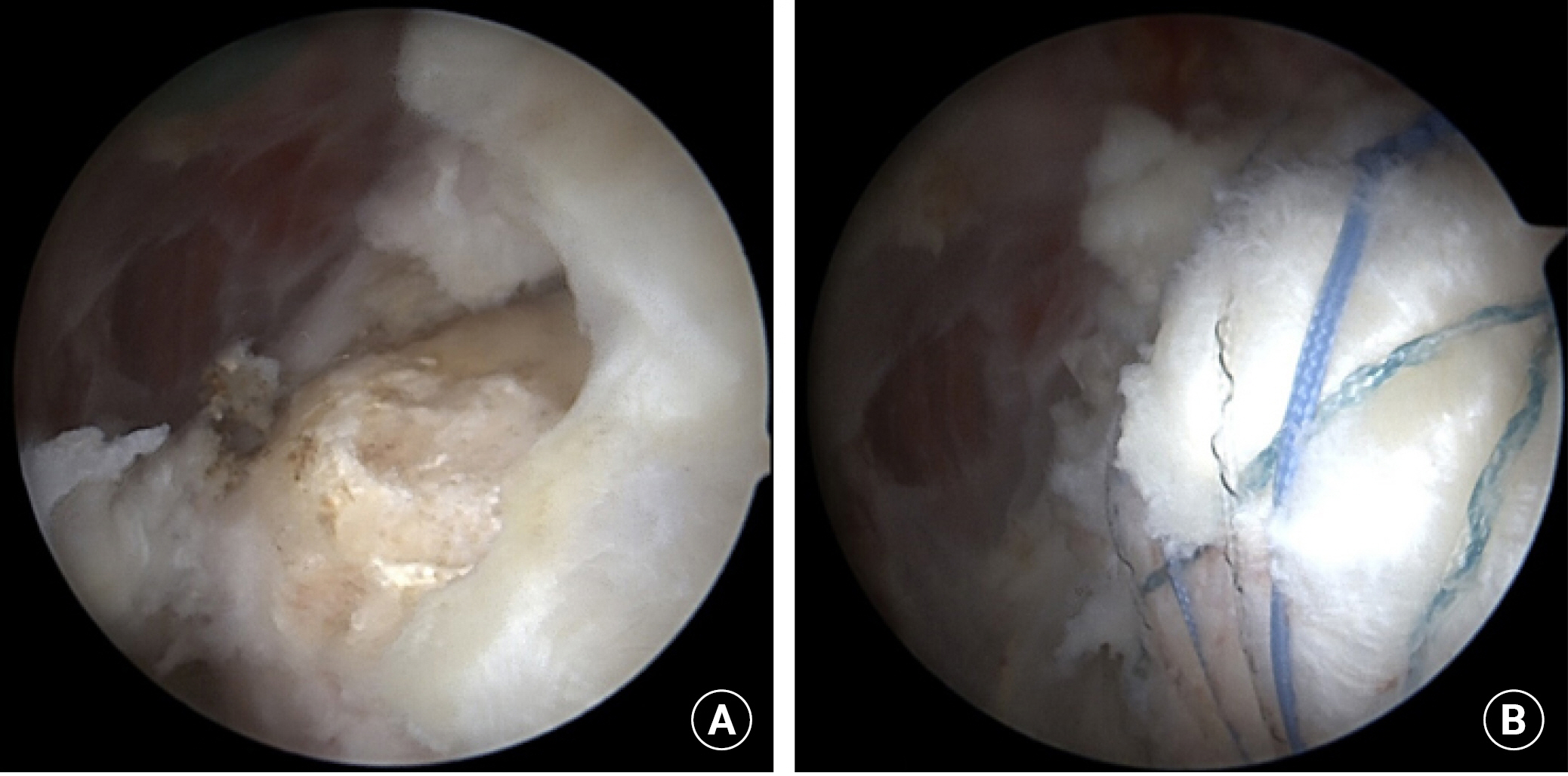
Double-row suture bridge repair using conventional suture anchors. (A) Before repair, torn tendons of supraspinatus and infraspinatus were retracted, and the greater tuberosity was exposed. (B) Torn tendons were reduced to the tip of the greater tuberosity and fixed by the double-row suture bridge technique with two conventional medial suture anchors and two knotless lateral suture anchors.
ToR was performed using a transosseous tunneling system (TransOS Tunneler, Tensor Surgical) (Fig. 3A) with high-strength suture threads (FiberWire, Arthrex) retrieved from the conventional suture anchor (Corkscrew FT, Arthrex). However, the suture anchor itself was not inserted in the proximal humerus. First, two medial holes were created using a medial awl on the articular margin of the humeral head according to the anterior (anteromedial hole) and posterior (posteromedial hole) portion of the torn rotator cuff (Fig. 3B). Next, a distal hook of the TransOS Tunneler was inserted into the medial holes (Fig. 3C). Sequentially, a lateral awl, which attaches the guide suture at the tip, was inserted into the inner guide sleeve and advanced to make the anterolateral and posterolateral holes (Fig. 3D). The guide suture was then pulled automatically at the tip of the distal hook, and the lateral awl was disassembled from the tunneler. After removing all instruments, transosseous tunnels connecting the medial and lateral holes were made, and the guide suture remained in the anterior and posterior tunnels, respectively (Fig. 3E). Next, three suture threads were tied with a guide suture, and these suture materials were passed through the transosseous tunnel using the suture relay technique (Fig. 3F and G).
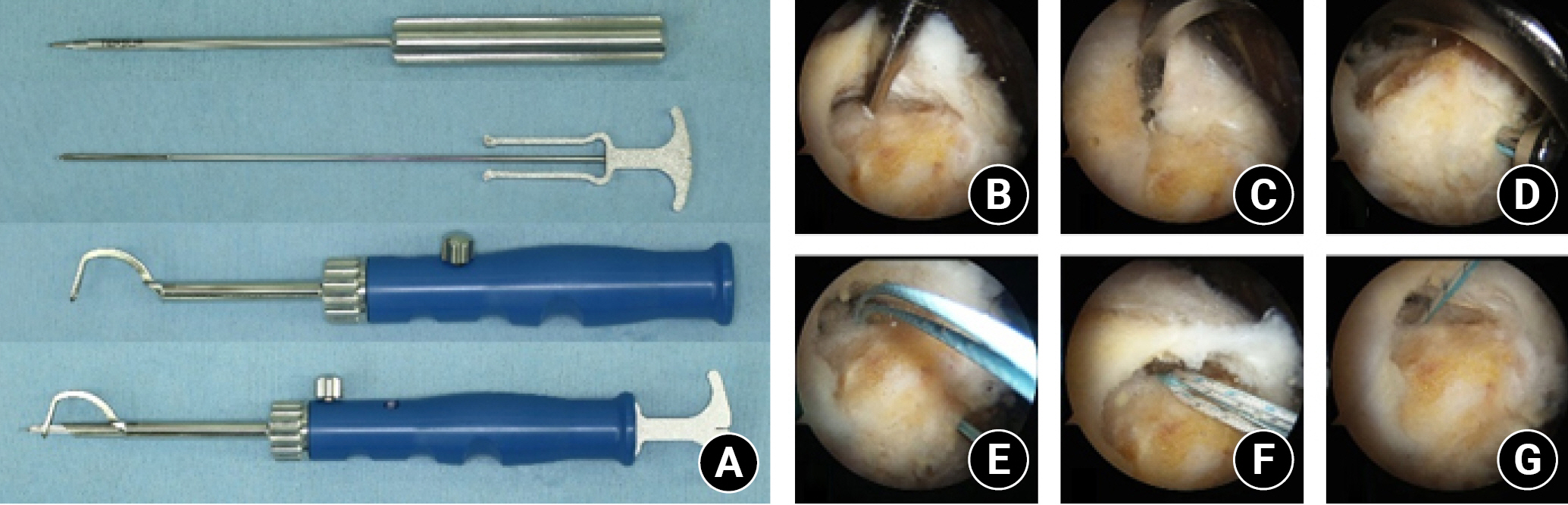
Transosseous tunneling system and tunnel-making process. (A) The transosseous tunneling system comprises three components, arranged from top to bottom: a medial awl, a lateral awl, and a tunneler with a hook. To use the system, the lateral awl is inserted into the inner guide of the tunneler with a hook and assembled together (bottom). (D) The lateral awl was assembled with the tunneler. A guide suture was attached at the tip of the lateral awl. The lateral awl was inserted until its tip touched the hook of the tunneler. (E) The lateral awl and the tunneler were retrieved sequentially. The guide suture was passed through the transosseous tunnel. (F, G) The suture material was inserted into the transosseous tunnel using the suture relay technique.
The three suture threads penetrated the anterior and posterior margin of the torn rotator cuff using the suture relay technique (Fig. 4A). Initially, the suture threads from anterolateral and posteromedial holes were fastened together and pulled out, resulting in the combination of two suture threads into one (Fig. 4B). Then, two suture threads from the medial holes were tied together, and the other side of the tied suture threads from the lateral holes were pulled and fastened together to create a horizontal matrix suture box (Fig. 4C). Subsequently, a square-shaped suture box was made using two simple vertical sutures from the anterior and posterior tunnels (Fig. 4D). Sequentially, the first penetrated suture threads from anterolateral and posteromedial holes were tied to make an X-shaped suture (Fig. 4E). Finally, an X-box shaped double-row suture bridge was created using the ToR technique (Fig. 4F).
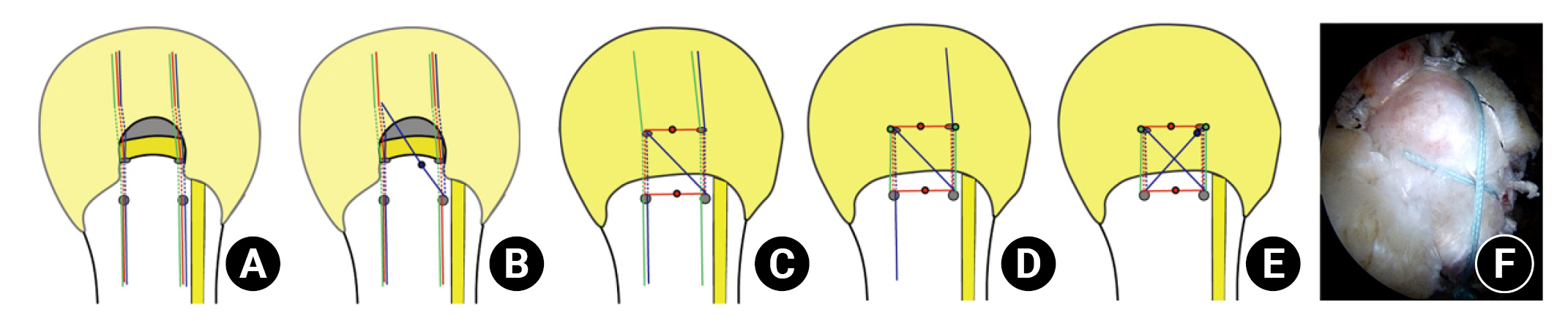
Anchorless X-box-shaped double-row suture bridge technique. (A) Three suture threads penetrated the anterior and posterior margins of the torn rotator cuff. (B) The suture threads from the anterolateral and posteromedial holes were tied together (blue dot). The tied suture threads were sequentially retracted to make two suture threads into one. (C) Two pairs of suture threads were tied together from the medial holes (medial red dot), and the torn tendon was pressed down by a horizontal matrix suture completed by tying the suture threads from the lateral holes (lateral red dot). (D) The anterior and posterior portions of the torn tendon were pressed down with two simple vertical sutures (green dots). (E) The penetrated suture thread (blue) was initially tied to make an X-shaped suture (blue dot). (F) Configuration of an X-box-shaped double-row suture bridge.
Patients wore an abduction brace for 5 weeks postoperatively. Active assisted ROM exercises were initiated after the immobilization period. Furthermore, strengthening exercises, including forward flexion, abduction, and external and internal rotation using resistance rubber bands, were introduced 3 months postoperatively. Various activities, including sports, were usually allowed 6 months postoperatively.
Statistical Analysis
All statistical analyses were performed using R ver. 4.0.5 (The R Foundation for Statistical Computing) and RStudio version 1.4.1106 (RStudio Inc.).
The intraoperative implant failure rate was compared among the surgical techniques used in the overall study population. Other radiological and functional outcomes were compared after the exclusion of cases with intraoperative implant failure. After exclusion of the cases with intraoperative implant failure, PSM was performed using sex, age at time of surgery, hand dominance of the operation side (dominant or non-dominant arm), tear size, and systemic BMD to minimize bias originating from confounders that could affect the local BMD of the proximal humerus [13-18]. PSM was conducted at a ratio of 1:3 (ToR:SA=1:3) to increase the statistical power.
Continuous variables were subjected to the Shapiro-Wilk normality test to determine their distribution, and the choice of parametric or nonparametric tests was based on the outcome of this test. Independent t-tests or Mann-Whitney U-tests were used to compare functional outcomes. Paired t-tests or Wilcoxon signed-rank tests were used to compare pre- and postoperative functional outcomes. Chi-square or Fisher’s exact test was conducted to compare intraoperative failure rate, retear rate, and peri-implant cyst formation rate. Intra- and inter-observer reliabilities were measured using Cronbach’s alpha test. Power analysis of statistical differences in the intraoperative implant failure rate, retear rate, and peri-implant cyst formation rate was performed. All statistical analyzes were two-sided, with a significance level of 0.05 and a power of 0.8.
RESULTS
Of the 286 consecutive ARCR cases, patients with a follow-up period of less than 2 years (n=61) and those who underwent concurrent subscapularis repair (n=89) were excluded. Of the remaining 136 patients, those whose bone was cut through by the suture material in the ToR group (n=2) and those with intraoperative suture anchor failure in the SA group (n=17) were analyzed only for the intraoperative failure rate and excluded from the analysis of functional and radiological outcomes. Therefore, 117 patients were enrolled for analysis and divided into two groups based on the repair technique used (23 in the ToR group and 94 in the SA group). After PSM, 76 patients (19 in the ToR group and 57 in the SA group) were finally evaluated for their radiological and functional outcomes. There were no significant differences in the matched variables (Table 1). The mean follow-up duration was 37.6±12.4 months and did not significantly differ between the two groups (P=0.286) (Table 1).
To evaluate the safety of the ToR technique, the intraoperative implant failure rate was calculated before PSM. The intraoperative implant failure rate in the ToR group (8%, 2/25) was not significantly different from that in the SA group (15.3%, 17/111; p=0.525, power=0.764). Two cases of intraoperative implant failure in the ToR group were treated by converting to SA, and all cases of intraoperative implant failure in the SA group were treated with metallic suture anchors with a larger diameter. The mean operation time in the cases with intraoperative implant failure (110.0±21.4 minutes) was significantly longer than that in cases without intraoperative implant failure (95.2±23.9 minutes, P=0.021). The systemic BMD measured using DXA was not significantly different according to the repair technique (ToR –0.9±1.3 vs. SA -1.4±1.0; P=0.101) or presence of intraoperative implant failure (implant failure -1.6±1.0 vs. non-failure -1.3±1.0; P=0.112).
To compare medical costs, the number of suture anchors used and operation time were evaluated. In the ToR group, two medial suture anchors were used to retrieve the high-strength suture threads, while two medial suture anchors and two lateral suture anchors were used in the SA group. Operation time was not significantly different between the ToR group (98.3±22.6 minutes) and the SA group (94.2±24.4 minutes, P=0.520).
Functional outcomes were improved postoperatively in both groups (all P<0.05) (Table 2). However, they were not significantly different at the pre-operative work-up and final follow-up according to the repair technique (all P>0.05) (Table 2). Fatty degeneration of each rotator cuff muscle was not significantly different between the two groups (all P>0.05) (Table 1). While the retear rate at the last follow-up in the ToR group (5.3%, 1/19) was lower than that in the SA group (19.3%, 11/57), the difference between the two groups was also not significant (P=0.274, power=0.977).
Furthermore, the intra- and inter-observer reliabilities for peri-implant cyst formation were calculated. Intra-observer reliabilities of both observers were 0.960 and 0.947, respectively (all P<0.001). Inter-observer reliability was 0.899 for the anteromedial suture anchor and 0.922 for the posteromedial suture anchor (all P<0.001). Since the intra- and inter-observer reliabilities presented excellent outcomes, mismatched results were re-established through a consensus between two observers. Peri-implant cyst formation was not observed in the ToR group (0.0%, 0/38); however, it was observed in 16.7% of the SA group (19/114). This difference was statistically significant (P=0.008, power >0.999).
In the SA group, four types of medial suture anchors were used (Table 3). Peri-implant cyst formation rate was significantly different according to the material characteristics of SA (P=0.007) (Table 2). Peri-implant cysts most frequently occurred in PEEK (50.0%, 2/4) and were not observed in the all-suture anchor (0.0%, 0/9) (Table 3). Four types of lateral knotless suture anchors were utilized in the SA group (Table 3). However, the peri-implant cyst formation rate around the lateral SAs could not be analyzed due to the metal artifact. This was because most of the lateral suture anchors used contained metallic portions (93.0%, 106/114).
DISCUSSION
Suture anchors enable ARCR; however, suture anchor-related problems, including peri-implant cyst formation, pull-out, or economic costs, have not been entirely resolved. [3-7] Therefore, this study was conducted to evaluate whether the ToR technique could overcome these suture anchor-related problems. In the pre-matched data of this study, the intraoperative implant failure rate was not significantly different between the ToR and SA groups. In addition, radiological and functional outcomes were improved postoperatively in both groups, and the outcomes were not significantly different according to repair technique. Furthermore, peri-implant cyst formation was not observed in the ToR group but was observed in 17.9% of the SA group.
One of the most important findings of this study was the peri-implant cyst formation rate. Previous studies on peri-implant cyst formation have reported that structural and/or functional outcomes did not vary according to the presence of peri-implant cysts [5-7]. However, decreased bone stock owing to the peri-implant cyst may provoke problems during revision surgery. In this study, peri-implant cyst formation was not observed in the ToR group, and the authors considered that the nonabsorbable nature of suture materials involved in the ToR technique might be the reason for less osteolytic reaction after rotator cuff repair [7]. While further studies with longer follow-up periods are required to elucidate correlations between the peri-implant cyst and structural and/or functional outcomes after ARCR, the absence of peri-implant cysts might be an advantage of the ToR technique.
Pull-out of the suture anchor is also an issue. Intraoperatively pulled out suture anchors can provoke bone defects larger than the diameter of the suture anchor [27]. Therefore, additional procedures are often required to manage bone defects. In this study, pulled out suture anchors were managed using metallic suture anchors with a larger diameter. Several biomechanical studies have demonstrated that suture anchors of large diameter presented higher pull-out strength [28,29]. Another method to manage pulled out suture anchors was the buddy anchor technique, which inserts a second anchor adjacent to the loose suture anchor. It could reinforce the pull-out strength with an interference fit between two suture anchors [30]. Suture anchor augmentation can be achieved by inserting bone cement into a bone defect site [31]. However, these techniques inevitably increase the number of suture anchors and/or operation time to insert additional suture anchors or cement curing time.
In this study, the intraoperative implant failure rate was not significantly different according to repair technique, and suture cut-through in the ToR group was managed with suture anchor insertion. Black et al. [32] reported the intraoperative implant failure rate of the ToR technique. In their study, suture cut-through of the bone during knot-tying occurred in two of 31 patients (6%), which was similar to the results of this study. They also used suture anchors to manage the suture cut-through of the bone and presented favorable clinical outcomes at the final follow-up. Physically, pressure is inversely proportional to the size of the area on which the force is applied. Considering the suture configuration, the pressure on the bone by the suture materials might be higher in the ToR technique due to the narrow surface area of the suture. However, in the conventional DRSB technique using suture anchors, the pressure is distributed to the suture anchors, and the surface area of the suture anchor is also larger than the surface area of the suture materials in the ToR technique. Therefore, the authors considered this the reason why the suture anchor could manage the suture cut-through of the ToR technique. To avoid suture cut-through, broader suture material might be helpful as it could decrease the pressure at the contact region with a wider surface area [33,34].
Interestingly, systemic BMD was not significantly different according to intraoperative implant failure. Osteoporosis is a systemic disease that depends on bone metabolism. Therefore, systemic osteoporosis could affect the BMD of the proximal humerus. However, the limitation of the current diagnostic system for systemic osteoporosis is that the examination is limited to the lumbar spine and the hip. Therefore, systemic BMD might underestimate local disuse osteoporosis of the proximal humerus [35]. Several previous studies have argued that systemic BMD could not represent the local BMD of the humerus [13-15].
However, the measurement of local BMD of the proximal humerus is not commonly performed in clinical settings due to the absence of standardized methods to diagnose local osteoporosis of the proximal humerus. Although the local proximal humeral BMD could not be measured in this study, the authors performed PSM with the variables that could affect the local BMD of the proximal humerus (sex, age, hand dominance, tear size, and systemic BMD) to minimize the bias [13-18].
While healthcare-related costs are greatly affected by each country’s policies, they have gradually increased with the development of technology over the past several decades [36-38]. Regarding ARCR, the total sum of used suture anchors is correlated with higher supply costs [3,4,39]. The transosseous tunneling system used in this study was designed to be reusable; therefore, it might reduce suture anchor-related costs [40]. In South Korea, government-regulated medical costs, including for suture threads, resulted in high-strength sutures not being appropriately priced. As a result, the authors were unable to use high-strength suture threads and had to retrieve them from the two conventional suture anchors in the ToR group. Although the authors could not compare direct medical costs between the ToR and SA groups due to these government regulations, the exclusion of lateral suture anchors in the ToR group may result in lower costs when compared to the SA group. Furthermore, there was no significant difference in operation time between the two groups, which is another cost-related factor to consider.
This study has several limitations. First, the possibility of selection bias cannot be excluded due to the retrospective manner of this study. Additionally, the authors could not guarantee that the possibility of selection bias was fully excluded during the selection of surgical treatment method. Local BMD of the proximal humerus could affect the provocation of the intraoperative implant failure and/or peri-implant cyst formation. Although systemic BMD presented no statistical differences between the ToR and SA groups in this study population including or excluding the cases with intraoperative implant failure, several previous studies have argued that systemic BMD does not represent the local BMD of the proximal humerus [13-15]. However, measuring the local BMD of the proximal humerus is not routinely conducted in clinical situations due to a lack of standards to diagnose local osteoporosis of the proximal humerus. To overcome this limitation, further studies using a ToR technique with the measurement of the local BMD of the proximal humerus are necessary. A small sample size might be another limitation; however, the statistical power of essential variables, including intraoperative implant failure rate, retear rate, and peri-implant cyst formation rate, was calculated. Therefore, the authors suggest that the results of this study have statistical value. Furthermore, the authors did not analyze the reason for the intraoperative implant failure. However, it was estimated as a multifactorial complication with various causes, and the type of suture anchors used in this study varied. To elucidate this issue, comparative studies regarding the causal relationship of implant failure rate according to surgical techniques might be necessary. Additionally, peri-implant cyst formation was simplified to binary values. Several previous studies have presented a classification system for peri-implant cysts around the suture anchor [5-7].
However, this classification system could not be used in this study due to the morphologic differences between the suture threads and the suture anchor. Furthermore, as there were no peri-implant cyst formations in the ToR group, the suggestion of a new classification system for the ToR group based on functional or radiological outcomes was not possible. Finally, medical costs between the two groups were not directly compared, because a direct comparison the medical costs according to surgical technique was likely to be biased by current government policies. It is important to note that this study did not aim to compare medical costs according to surgical technique, and further studies are needed to evaluate the cost-benefit of different surgical techniques to overcome this limitation.
CONCLUSIONS
The ToR technique presented clinical outcomes comparable to those of SA. However, considering the prospect of potential additional surgeries, the absence of peri-implant cyst formation might be an advantage of the ToR technique. Furthermore, ToR might reduce the medical costs related to suture anchors and, thereby, could be a viable option for ARCR.
Notes
Author contributions
Conceptualization: HJJ, SMR, JHO. Data curation: HJJ, JSL, YKK, SMR. Formal analysis: HJJ, JSL, YKK, SMR. Investigation: HJJ, JHO. Methodology: HJJ. Supervision: JHO. Writing-original draft: HJJ. Writing-review & editing: HJJ, JHO.
Conflict of interest
None.
Funding
None.
Data availability
Contact the corresponding author for data availability.
Acknowledgments
None.