Prosthetic resurfacing of engaging posterior capitellar defects in recurrent posterolateral rotatory instability of the elbow
Article information
Abstract
Background
Posterolateral rotatory instability (PLRI) is a common mechanism of recurrent elbow instability. While the essential lesion is a deficiency in the lateral ulnar collateral ligament (LUCL), there are often associated concomitant bony lesions, such as an Osborne-Cotterill lesions (posterior capitellar fractures) and marginal radial head fractures, that compromise stability. Currently, there is no standard treatment for posterior capitellar deficiency associated with recurrent PLRI.
Methods
We conducted a retrospective review of five patients with recurrent PLRI of the elbow associated with a posterior capitellar impaction fracture engaging with the radial head during normal range of motion. The patients were treated surgically with LUCL reconstruction or repair and off-label reconstruction of the capitellar joint surface using a small metal prosthesis designed for metatarsal head resurfacing (HemiCAP toe classic).
Results
Five patients (three adolescent males, two adult females) were treated between 2007 and 2018. At a median follow-up of 5 years, all patients had complete relief of their symptomatic instability. No patients had pain at rest, but two patients had mild pain (visual analog scale 1–3) during physical activity. Three patients rated their elbow as normal, one as almost normal, and one as greatly improved. On short-term radiographic follow-up there were no signs of implant loosening. None of the patients needed reoperation.
Conclusions
Recurrent PLRI of the elbow associated with an engaging posterior capitellar lesion can be treated successfully by LUCL reconstruction and repair and filling of the capitellar defect with a metal prosthesis. This treatment option has excellent clinical results in the short-medium term.
Level of evidence
IV.
INTRODUCTION
Posterolateral rotatory instability (PLRI) has been described in recent decades as a leading mechanism of recurrent elbow instability [1,2]. Insufficiency of the lateral ulnar collateral ligament (LUCL) is an essential lesion in PLRI, and is a primary constraint of the elbow [3]. Nonetheless, compromised movement due to several constraints may be present simultaneously, such as concomitant deficiency of the coronoid, radial head, or capitellum.
Several bony lesions have been associated with recurrent PLRI. The most common is a dual lesion of the coronoid process and the radial head ("terrible triad"), and these lesions are well recognized and treated in the acute setting. Another dual lesion, concomitant fracture of the radial head and the posterior capitellum, resembling engaging Hill-Sachs and Bankart lesions of the shoulder, was described in 1966 by Osborne and Cotterill [4] (Fig. 1). While they described these lesions as being associated with recurrent elbow instability, it was later shown that they occur with PLRI [5,6]. The posterior capitellar fracture was subsequently recognized to occur either due to impaction [7] or a shear fracture creating a loose bone fragment [8]. Nonetheless, both produce the same biomechanical effect of reduced joint-contact area and stability.

Patient 2: computed tomography scan demonstrating the Osborne-Cotterill dual lesion. (A) Inferior view of the distal humerus depicting a compression fracture of the posterior capitellum (“Osborne-Cotterill lesion”). (B) Proximal view of the radial head depicting a marginal defect on the antero-lateral quadrant. (C) Sagittal view showing the radial head about to engage with the capitellar defect with the elbow nearing full extension. Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Reports on the treatment of recurrent PLRI associated with posterior capitellar deficiency are scarce, and currently only nine published cases were found in a literature review. In all published cases, the LUCL was reconstructed, but they differ in regarding to capitellar lesion treatment: three cases of bony fragment removal [8]; two cases where the capitellar lesion was not addressed [9,10]; one case of osseous reconstruction using a bone graft [10]; one case using an osteochondral autograft transfer (OATS) [11]; and one case using an osteochondral allograft transfer [12].
The senior author of this study has implemented an off-label use of a small prosthesis designed for metatarsal head resurfacing (HemiCAP toe classic, Arthrosurface) to reconstruct the posterior capitellar surface for recurrent PLRI with an engaging, large, posterior capitellar defect. The purpose of this study is to report the indications and results of this experience.
METHODS
This retrospective, chart-review study was conducted under the Mayo Clinic Institutional Review Board protocol (No. 11-002988), in which waiver of participant consent was granted. All cases (n=5) of elbow PLRI associated with a posterior capitellar deficiency that were treated using a HemiCAP toe prosthesis from 2007 to 2018 were reviewed. To be included in the study, all patients must have had at least 1 year of follow-up.
Patient Description
Patient 1, an 18-year-old right-hand-dominant male, presented 10 months following a fall on his outstretched left hand resulting in a simple elbow dislocation that he was able to self-reduce. He complained of recurrent painful popping in the lateral elbow when extending and supinating. On physical examination, he had marfanoid habitus and generalized joint laxity. Range of motion (ROM) was full and symmetrical, with –5° to 165° flexion arc and 90°/90° pronation/supination. He had a grossly positive posterolateral rotatory drawer test and a positive lateral pivot-shift test. X-ray and computed tomography (CT) scan showed lateral and posterolateral capitellar deficiency accompanied by anteromedial coronoid deficiency, and a small marginal radial head defect.
Patient 2, a 52-year-old right-hand-dominant female, presented 6 months after a fall from her bicycle onto her outstretched left hand. A radiograph showed no sign of fracture, and she underwent 2 weeks of immobilization. Following cast removal, she developed symptoms of elbow instability. An magnetic resonance imaging (MRI) showed LUCL injury, and she was treated with another 4 weeks of immobilization. She continued to suffer from feelings of recurrent subluxations and posterolateral pain. On physical examination, she had full ROM and a positive posterolateral rotatory drawer test without significant apprehension. A CT scan revealed a posterior capitellar impaction defect, a small marginal radial head defect, loose bodies, and a bony avulsion of the ulnar attachment of the LUCL (Fig. 1).
Patient 3, a 44-year-old right-hand-dominant female, originally fell on her left outstretched hand 1.5 years prior to presentation at our institution. She suffered avulsion of the origin of the LUCL. Three months following the injury she underwent LUCL reconstruction. Following surgery, she continued having recurrent mechanical symptoms of popping, feelings of elbow subluxation, and developed elbow pain during extension. On physical examination, she had full bilateral ROM, positive posterolateral rotatory drawer and lateral pivot-shift tests, and a positive posterolateral rotatory apprehension test. Radiographs and CT showed a posterior capitellar impaction defect.
Patient 4, a 12-year-old right-hand-dominant male, presented 6 months following a hyperextension injury to the elbow during wrestling. He was treated with a short period of immobilization, however, was unable to regain pain-free function and suffered from painful locking episodes. CT showed a comminuted posterior capitellar impaction defect and a Salter-Harris type-III radial head fracture nonunion with multiple loose bodies. He was treated with arthroscopic debridement and removal of loose bodies. After going back to wrestling 6 weeks following surgery, he developed episodes of painful elbow subluxation. On physical examination 4 months following arthroscopy, he had full ROM, posterolateral tenderness, slight varus and valgus laxity, and a positive posterolateral rotatory drawer test. A repeat CT scan showed the same impaction defect of the capitellum and enlargement of the non-united radial head fragment (Fig. 2).

Patient 4. (A) Three-dimensional reconstructions of computed tomography (CT) scan demonstrating posterior capitellum fracture with some comminution, and a Salter-Harris type-3 radial head fracture. (B) Preoperative CT showing the lesions almost engaging during extension. (C) CT scan performed 6 months after surgery shows restoration of the articular constraint against radial dislocation. Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Patient 5, a 17-year-old left-hand-dominant male, presented 3 months following an episode of an acute feeling of elbow subluxation and pain, which happened during a drill that included walking on all fours. After the initial episode, he experienced recurrent episodes of similar symptoms. His history is significant for a capitellar fracture of the lateral rim and a posterior capitellum that was treated surgically a year prior by removal of the loose fragments and microfracture of the capitellum. He also had a coronoid fracture 5 years prior that was treated surgically by open reduction and internal fixation. On physical examination, he had full ROM, a positive posterolateral rotatory drawer test, and a clunk that occurred at about 10º–20º of pronation. CT showed slight posterior subluxation of the radial head with a significant defect of the posterior and lateral rim of the capitellum and a slightly hypoplastic radial notch.
Surgical Indication
The indication for performing posterior capitellar reconstruction is a posterior capitellar lesion in the context of symptomatic PLRI, with engagement of the radial head in the presence of capitellar deficiency during normal range of motion. This engagement is confirmed twice: (1) during examination under fluoroscopy at the beginning of the surgery and (2) by direct examination during surgery, once the radiocapitellar joint is opened.
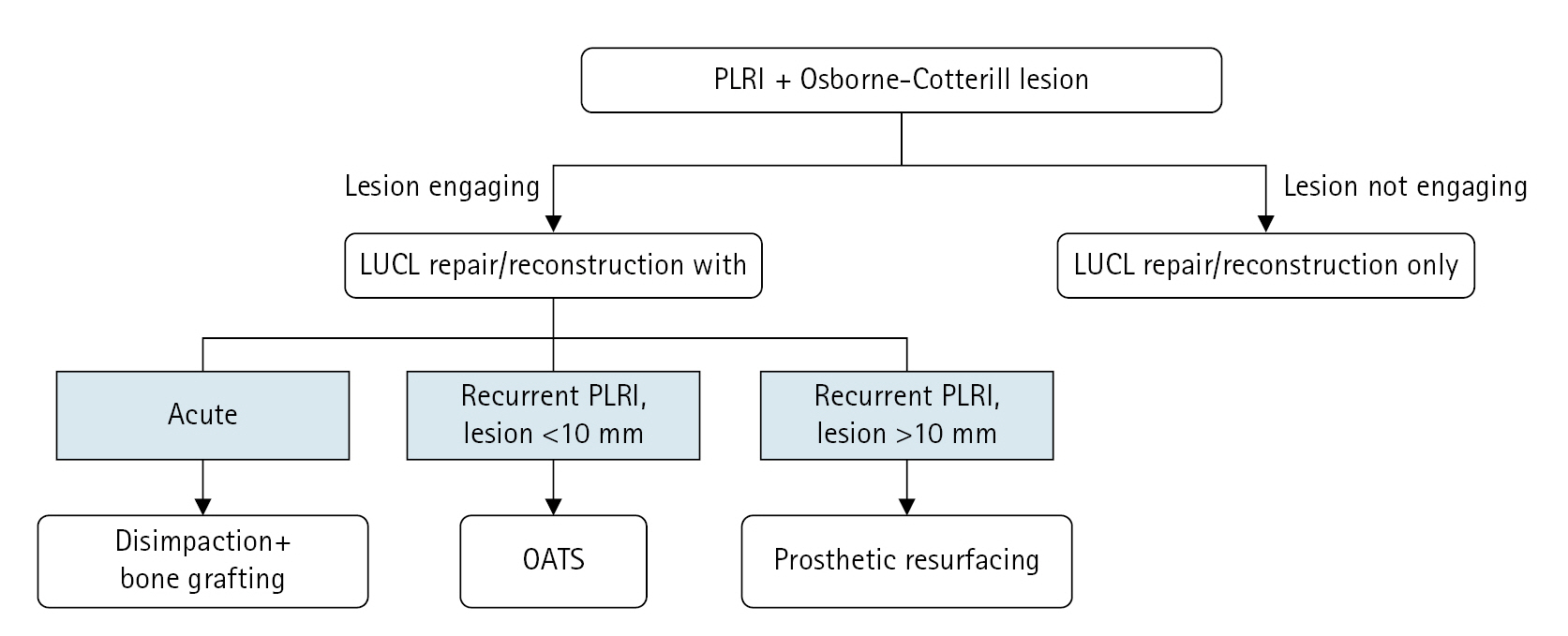
After the decision is made that posterior capitellar reconstruction is needed, several surgical options exist in our practice: (1) dis-impaction plus bone grafting, (2) OATS, or (3) prosthetic reconstruction. While the first option is viable for acute injury (see case 1 in [10]), it may not be feasible in chronic or recurrent cases. The decision between OATS and prosthetic reconstruction is made predominantly by considering the size of the deficient area: if it is 10 mm or smaller in diameter, a 10-mm osteochondral allograft cylinder is preferred. If it is larger than 10 mm in diameter, a prosthetic reconstruction is preferred (Fig. 3). All patients received a thorough explanation of the potential risks and benefits associated with the off-label use of a toe prosthesis before consenting to surgery.
Surgical Technique
The following description closely follows the surgical course for patients 2–5. Patient 1, who had combined lateral and medial instability, underwent a box-loop ligament reconstruction as previously published [13]. The box-loop was indicated for combined medial and lateral collateral ligament deficiency.
Following anesthesia, an examination under fluoroscopy was performed wherein a positive posterolateral rotatory drawer test was elicited and the radial head with capitellar deficiency during normal range of motion was engaged. A lateral elbow approach using the anconeus interval was used, and once the radiocapitellar joint was clearly visible, it was reaffirmed that the radial head was engaging in the capitellar defect during normal range of motion, and if so, the decision is made to perform posterior capitellar reconstruction.
The standard technique detailed in the Arthrosurface HemiCAP technique guide [14] was used, wherein prosthesis sizing is determined intraoperatively, choosing the size and offset that fit best without creating a significant overhang. Usually, a 12-mm prosthesis with an offset of 2 mm×3 mm has the best fit, although the orientation may differ: in some patients the long radius of the prosthesis is placed along the longitudinal axis of the capitellum, whereas in others, the prosthesis is placed with the shorter radius along the longitudinal axis of the capitellum to prevent a sharp edge hitting against the radial head. The prosthesis is then placed about 1-mm deep into the surrounding cartilage surface (Fig. 4). At this point, the prosthesis should articulate well with the radial head and prevent it from slipping.
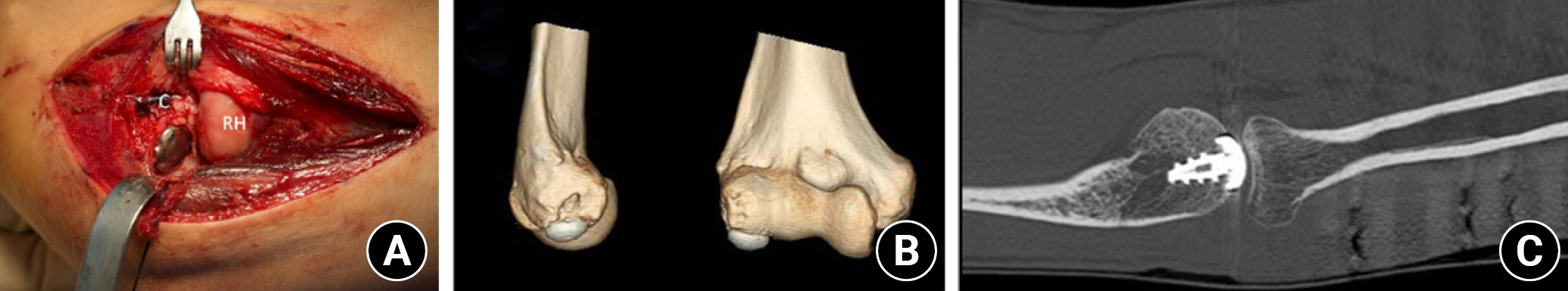
(A) Intraoperative photograph of the prosthesis in place (patient 5). Note its placement minimally deep into the surrounding cartilage surface. (B) A three-dimensional reconstructed computed tomography (CT) scan of the same patient 7 months following surgery. The prosthesis may look prominent, but that is because CT reconstruction does not include the cartilage surface. (C) Although the radial head is mildly posteriorly subluxated, the restored articular constraint prevents dislocation. C: capitellum, RH: radial head. Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.
Next, a ligament reconstruction/repair was performed. The standard tunnels in the humerus used for LUCL allograft reconstruction is not always usable because of the implant’s stem, and the surgeon should be prepared to modify them as needed. Postoperatively, patients were immobilized at 90º of flexion for 3 weeks (unless other components of the procedure necessitated a longer immobilization), followed by a removable splint for 3 more weeks, which the patient was instructed to remove multiple times per day for overhead active range of motion exercises. The removable splint was later used for another 6 weeks with gradual weaning. All patients were then referred to physiotherapy.
Outcome Measures
Outcomes included summary outcome determination (SOD) category and score [15]; visual analog scale for pain at rest, during or following physical activity, and at its worst; elbow stability assessed subjectively and by physical examination using the posterolateral rotatory drawer test; and range of motion. Complications or reoperation were recorded. Outcome data were obtained during routine follow-up visits and completed with mail-in or telephone questionnaires. Range of motion was obtained during clinic visits or by using photography-based goniometry [16].
A preoperative CT scan in full elbow extension was performed for all patients, and CT images was evaluated for bony lesions or malformations. Lesion locations were described from the sagittal view of the mid capitellum using a perfect circle technique and measuring the angle of the involved capitellum (Fig. 5), with the longitudinal axis of the humerus marking the 0 angle. The latest follow-up radiographs of the elbow were reviewed for signs of prosthesis loosening and radiocapitellar degenerative changes using the Broberg and Morrey osteoarthritis scale [17].

Lesion location measurement was performed on sagittal computed tomography of the mid-capitellum. First, a circle was fitted to match the capitellum. Second, the anterior humeral line was drawn (using a different slice) to indicate the 0 angle. Then, using an on-screen protractor, the anterior and posterior borders of the lesion were marked, and their location was measured. Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.
RESULTS
During the study period, five patients (three adolescent males aged 12, 17, and 18, and two adult females aged 44 and 52) underwent posterior capitellar reconstruction using the HemiCAP toe prosthesis, and none were lost to follow-up. The non-dominant side was involved in all five cases. In all patients, the indication for surgery was recurrent PLRI of the elbow and the decision to augment the capitellar surface was due to a defect in the posterior capitellum engaging with the radial head during normal range of motion (as evidenced by CT scan, and later confirmed during operation). Patients’ backgrounds and symptoms are summarized in Table 1. All patients underwent LUCL repair, if feasible, or reconstruction, and posterior capitellar reconstruction using the HemiCAP prosthesis and additional components as needed (Table 1). Median clinical follow-up was 5 years (range, 1–6 years). Patients’ clinical results are summarized in Table 2.
Median radiographic follow-up was 4.5 years (range, 3 months–7.5 years). Lesion locations are described in Table 3. Only one patient (patient 1) showed mild progression of joint arthritis, from 0 in preoperative imaging to 1 at the final follow-up, and the rest of the patients had no signs of joint arthritis. There were no signs of prosthesis loosening. There were no complications following surgery and at the final follow-up none of the patients needed a reintervention.
DISCUSSION
This study shows excellent clinical outcomes for treatment of recurrent elbow PLRI associated with an engaging posterior capitellar defect. These results are achieved by addressing both instability components—reconstructing/repairing the LUCL and reconstructing the bony capitellar lesion—by off-label use of a small metal resurfacing prosthesis designed for toe hemiarthroplasty. There were no apparent complications or problems related to the use of the metallic prosthesis. This is novel because the current literature includes mostly case reports and lacks concise methodology for dealing with such pathologies.
The primary goal of this study is to bring attention to the need to meticulously look for and assess the clinical significance of bony lesions before performing a lateral elbow stabilizing surgery. Mild bony lesions could be easily missed or overlooked, especially if the preoperative imaging includes only simple X-ray and MRI and does not include CT with three-dimensional reconstruction [18]. We believe such an oversight may be a leading cause for failure of LUCL reconstruction surgery.
Osborne and Cotterill [4] noted the similarity between Hill-Sachs and Bankart lesions of the shoulder and the posterior capitellar and radial head lesions of the elbow. They argue that these lesions represent the same biomechanical pathology—bony lesions to both sides of a joint, decreasing bony restraint to dislocation [7], and causing recurrent joint instability. To treat shoulder instability, once a certain size of bone deficiency is exceeded, no soft tissue procedure can restore stability and a restoration of bony restraint is needed. We believe the same logic should be implemented for the radiocapitellar joint (Figs. 2 and 3).
Osborne and Cotterill have described the lesions associated with recurrent elbow instability as posterior capitellar defect, incomplete healing of the lateral collateral ligament and posterolateral capsule, and medial collateral ligament laxity and possible "shovel-like defect" in the radial head. Later, the term “Osborne-Cotterill lesion” was proposed to define the posterior capitellar lesion associated with PLRI [6,19]. The impaction involves a variable amount of the inferior (distal) capitellar articular surface and extends posteriorly into the non-articular surface.
In this small series, all patients suffered from an Osborne-Cotterill lesion, but only three had concomitant radial head lesions, and of those, only one was addressed during surgery. In most cases, the radial head lesions associated with recurrent PLRI are small marginal defects in the antero-lateral quadrant of the radial head when the forearm is supinated (Fig. 1), and as Osborn and Cotterill suggested, may be caused by recurrent dislocation of the radial head [4]. In one case in this series where the radial head was treated, the lesion was not a marginal deficiency but a non-union of a Salter-Harris type-3 fracture (Fig. 2).
Published findings regarding treatment for recurrent PLRI associated with Osborne-Cotterill lesions are scarce, and treatment options are limited: Jeon et al. [8] reported four cases, all of which underwent LUCL reconstruction, but only one case was augmented by “osseous reconstruction” of the capitellum using bone graft. One of the three patients who was not treated for the bony lesion had recurrent moderate instability, and the rest had excellent results. Clark et al. [11] reported one case of a 19-year-old woman who underwent LUCL reconstruction and osteochondral autograft transplantation for a capitellar lesion, using a 10-mm osteochondral cylinder. After 3 years, she had excellent clinical and radiographic results. Shukla and O’Driscoll [10] reported one case of a 35-year-old woman who failed conservative treatment for acute PLRI associated with a posterior capitellar impaction fracture and LUCL avulsion at the supinator crest. Eighteen weeks following her initial injury she underwent LUCL reconstruction without addressing the capitellar fracture. At 12 months follow-up she had no recurrent instability [10]. Patiño et al. [9] reported one case of a 23-year-old man, for which LUCL reconstruction and capitellar bone defect grafting and osteosynthesis were planned. During surgery, following LUCL reconstruction, the lesion was no longer engaging and so the decision was made to forego its treatment. After 2 years of follow-up, the patient had excellent clinical results [9]. Recently, Lee et al. [12] reported one case of a 23-year-old woman whose LUCL repair included internal bracing and osteochondral allograft transplantation using a 10-mm cylinder. After 2 years, she had excellent clinical and radiographic results [12].
Given these results, where most patients were not treated for a capitellar lesion, one might think that this is not necessary [9]. However, all but the last two published cases did not specify whether the capitellar lesion was engaging the radial head. It might be the case that most Osborne-Cotterill lesions are small, do not engage the radial head during the normal range of motion, and could be left untreated during ligament reconstruction. However, we believe that failure to detect and evaluate the large, clinically relevant cases, and address them during surgery, would probably lead to treatment failure. In the only study thus far describing patients who failed LUCL reconstruction surgery, 64% (7/11) had an osseous defect in the capitellum not addressed in the original operation [20].
Due to the lack of specific prosthesis designed for posterior capitellar reconstruction/resurfacing, it was decided to implement an off-label use of a prosthesis designed and approved for toe hemiarthroplasty. A literature review examining the outcomes of this prosthesis for its original indication found two long-term follow-up studies: Hilario et al. [21] reported results from 45/59 procedures. After a mean follow-up of 10 years, only one implant was removed (98% 10-year survivorship). Mermerkaya et al. [22] reported results from 57 patients, where after a mean follow-up of 7 years, seven cases (12%) underwent revision to arthrodesis. Nonetheless, all seven cases had early failure of the resurfacing surgery (within 1 year), and not a failure of the implant per se. Neither study reported any implant loosening or progressive joint arthrosis.
First, this study suffers from the common limitations of a retrospective case series, notably the small number of patients and lack of comparison with other treatment options. The capitellar defects in these patients were treated based on concern for their likelihood of causing failure of soft tissue reconstruction alone due to the engagement of the radial head into the capitellar defect during elbow extension, but prior to LUCL reconstruction. We do not know how the patients would have done had they been treated by ligament reconstruction only. Second, this study described off-label use of a prosthesis designed and approved for toe arthroplasty, which is not approved by the Food and Drug Administration (FDA) for use in the distal humerus. Unfortunately, there are no FDA-approved resurfacing prostheses designed specifically for the capitellum or the distal humerus. There is currently one published case implementing the same toe prosthesis for resurfacing of the humeral trochlea [23]. One should consider the potential hazards of off-label use of products before considering this treatment. Third, some patients had other concomitant procedures, limiting our ability to attribute the achieved clinical results to resurfacing and LUCL reconstruction alone. Fourth, radiographic characteristics (e.g., size and shape) of the capitellar lesions were not described, only their location. Future studies with a larger population are needed to better describe the characteristics of Osborne-Cotterill lesions associated with PLRI and possibly a threshold for surgical reconstruction. Fifth, though good results were achieved in this short-medium-term study, longer follow-up is needed to fully address potential complications such as prosthesis loosening and radial head arthrosis. Finally, this is a single-surgeon series, limiting the generalizability of the results.
CONCLUSIONS
Recurrent PLRI of the elbow associated with an engaging posterior capitellar impaction fracture can be treated by filling the bone defect with a small metal prosthesis at the time of LUCL reconstruction, which achieves excellent results in the short-medium term. We suspect that failure to recognize and treat engaging capitellar lesions may lead to a higher rate of treatment failure and recurrent instability.
Notes
Author contributions
Conceptualization: DR. Investigation: DR, JRL. Resources: SWO. Supervision: SWO. Visualization: DR. Writing – original draft: DR. Writing – review & editing: JRL, SWO.
Conflict of interest
None.
Funding
None.
Data availability
None.
Acknowledgments
None.