Rotator cuff degeneration and healing after rotator cuff repair
Article information
Abstract
Rotator cuff tear is a common shoulder injury that compromises both function and quality of life. Despite the prevalence of the injury and advancements in repair techniques, a significant percentage of these repairs fail. This review aims to explore the multifactorial reasons behind this failure, including the degenerative nature of the rotator cuff tendon, inherent and extrinsic factors, and the role of hypoxia in tissue degeneration. Additionally, it elucidates potential strategies for improving healing outcomes.
INTRODUCTION
The rotator cuff (RC), a crucial part of our musculoskeletal system, shows wear over time, often resulting in damage that significantly impacts mobility and quality of life [1,2]. These tears, both distressing and prevalent, have given rise to a burgeoning area of clinical and scientific research. The mechanisms of rotator cuff tear (RCT) healing, the intricate balance of collagen synthesis and degradation, and the influence of various risk factors on the outcome of healing are now more deeply understood, albeit still evolving areas of study [3].
Comprised predominantly of water (70%) and a dry mass of proteins (30%), the RC tendon primarily consists of collagen fibers, with type 1 collagen contributing to over 95% of the total. The other minor players, collagen types 3 and 5, account for the residual composition. Within this framework, tenocytes and tenoblasts emerge as the key cellular elements, constituting about 95% of the total cellular type. Intriguingly, a decrease in type 1 collagen is the signature feature of tendinopathy, a pathological condition affecting the tendon. The pivotal role of type I collagen in maintaining structural integrity is thus indicated [4].
However, even with the sophisticated understanding of the tendon composition and advanced surgical techniques, the likelihood of a retear post-repair remains high, and is reported to be anywhere from 13% to 94% [5-7]. Such a wide range of variability could be attributed to multiple factors, such as the inadequacy of diagnostic imaging, differing follow-up time points, and variable patient selection criteria. Furthermore, it is worth noting that retears are most common within the initial 6 to 26 weeks following arthroscopic rotator cuff repair, pointing to the crucial need for diligent postoperative management and ongoing research to further refine treatment protocols [8].
Therefore, exploring the complexity of RCT healing and strategies to enhance postoperative outcomes is a dynamic and imperative field of study. The intricate balance between collagen types, the role of cellular elements, and factors influencing retear rates need more thorough investigation for improved patient care and prognosis.
The complexity of RC degeneration necessitates a multifaceted approach for successful healing. There is a strong belief in the inherent ability of tendons to heal, even when moderately degenerated, irrespective of the suturing process. Yet, patient age and the size of the cuff tear play significant roles in the prognosis of healing. The need for institutional review board approval was waived because this is a review article.
TENDON DEGENERATION
Understanding the pathology and process of degeneration is crucial to unraveling the complex puzzle that is RC healing. In tendon degeneration, there is a marked shift in the collagen concentration, specifically to a higher prevalence of type III collagen. This type III collagen forms thin, reticular fibers, which are unfortunately more vulnerable to lesions, particularly when compared to the more robust type I collagen [9]. Intriguingly, these shifts in the tendon's composition and structural arrangement transpire in the absence of inflammation, leading to the condition recognized as a degenerated cuff [3].
Degenerated cuff can be characterized as a multifaceted landscape of chondroid metaplasia, fatty infiltration, and myxoid change. It is not uncommon to spot regions of dystrophic calcification, adding to the complexity of the pathology [10]. This degenerative process of the RC tendon is conceptualized in three distinct stages in a review article by Lewis [11]. The initial stage is denoted by reactive tendinopathy. Here, there is a transformation in the tenocytes, as they assume a more chondroid shape owing to the deposition of glycosaminoglycans. However, collagen integrity is, for the most part, preserved, although there may be some evidence of longitudinal separation. As the pathology progresses, the second stage of tendon disrepair is defined by extensive areas of swelling, evident tendon degeneration, and hypoechoic regions visible on ultrasound imaging that correlate with disorganization of the extracellular matrix. This stage is also characterized by an increase in the number of chondrocytes, as well as some myofibroblasts. This myofibroblast population surge results in a marked boost in proteoglycan synthesis, leading to separation of collagen fibers. The third and final stage manifests as degenerative tendinopathy, where tendon degeneration is paramount. In this stage, regions of apoptosis are identifiable, and the capacity for reversibility of pathological changes is significantly reduced. This stage witnesses notable heterogeneity of the matrix. For the RC, this stage is associated with significant structural failure, making this the most concerning phase in terms of potential recovery and healing.
Movin score, a semiquantitative histologic score, is used to diagnose degenerative tendons and assess their severity. It is derived from parameters like fiber structure, rounding of the nuclei, fiber arrangement, regional variations in cellularity, increased vascularity, decreased collagen stainability, and glycosaminoglycans content, without implying its decrease. This score has a range from 0 representing a normal tendon to 24 signifying a severe abnormality [12]. Similarly, the Bonar score is also used and considers variables such as tenocytes, ground substance, collagen, and vascularity. It ranges from 0 to 12, where 0 symbolizes a normal tendon, and 12 a severe abnormality [12]. A study by Sethi et al. [13] showcased pictures of a normal RC with a low Bonar score of about 3 and a degenerated cuff with a score of 12. The degenerated tissue exhibited increased vascularity, a marked separation of collagen fibers, and rounded, large tenocytes. However the degree of tendinopathy with Bonar score did not correlate with the gross appearance of the RC tendon, patient outcome, or RC healing in medium sized full-thickness RCTs. The extent of tendon degeneration can vary considerably based on degradation location and the state of the tendon, and is generally found to be more prominent in the supraspinatus tendon. Interestingly, in terms of cellularity, fiber organization, and nuclei shape, similar degrees of degeneration were noted in both intact and torn tendons [14].
The question therefore arises: why does healthy tissue degenerate? Tendon degeneration is, to some extent, a physiological aging process linked to the individual's age. It's important to emphasize that the discussion around pathological tendon degeneration primarily concerns degeneration that occurs in young or middle-aged patients, and not just the aging population [15]. Increasing age has been associated with higher rates of retears [16]. A systematic review and meta-analysis study by Longo et al. [17] found a retear rate of 14.4% in individuals under 60 years, which escalated to 24.3% in those over 60 years. Age has been linked with retears because of its association with fatty infiltration, a critical point of consideration in the ongoing research on RCT healing.
EXTRINSIC FACTORS
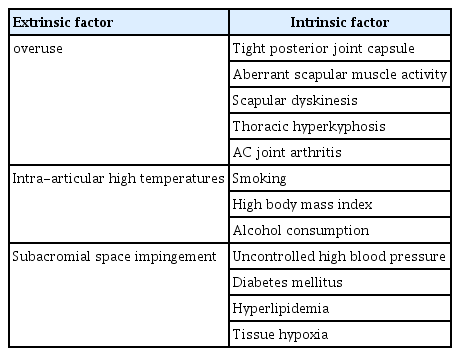
Understanding the intricacies of RCT healing necessitates an in-depth exploration of all relevant factors that influence this process. Both intrinsic and extrinsic factors, as well as the debatable concept of overuse, play significant roles in the progression and healing of these injuries (Table 1). Starting with the controversial topic of overuse, the traditional paradigm suggests that excessive use of the RC might lead to degeneration and subsequent tearing [18,19]. However, a work of research conducted on amputees who did not use prostheses provides an intriguing counterpoint [20]. In individuals with unilateral upper limb amputation, the healthy limb naturally incurs an increased workload during daily activity. Both shoulders of these patients were evaluated using magnetic resonance imaging, and the condition of the cuff was classified based on the Sugaya method [21]. Remarkably, the prevalence of RC degeneration or tearing was higher in the amputee side. This intriguing finding suggests that non-use, rather than overuse, might be a more substantial risk factor in the development of these injuries [20]. As early as 1970, Rathbun and Macnab [22] postulated that the muscle-tendon vascularity of the superoposterior cuff is “squeezed” when the arm is held in a position of neutral rotation and adduction. It is the position most commonly assumed by the amputee shoulder. Consequently, the RC on the amputee side might be poorly vascularized. This limited blood flow could play a critical role in tendon degeneration.
This brings us to another intriguing area of exploration, the possible effusion of synovial fluid into the RC during shoulder movement. Although it remains relatively unexplored, this phenomenon, if it occurs, could be profoundly affected by the limited mobility of the amputee limb, inhibiting the effusion of synovial fluid and possibly contributing to degeneration. Transitioning to the study of intra-articular high temperatures, it has been found that elevated temperatures within the joint can inflict serious damage to the collagen fibers of the extracellular matrix [23]. Experimental evidence has shown that at 37 °C, the stability of type I collagen derived from rat tail tendons is significantly diminished. As such, intra-articular high temperatures may well contribute to the progression of tear size, affecting the healing outcomes of RC injuries.
Finally, there is the once widely accepted notion that pathological narrowness of the subacromial space could be the principal cause of impingement, leading to tendon degeneration and tearing [24]. Bigliani and Levine [25] attributed the responsibility of impingement, degeneration, and tearing to the hooked shape of the acromion. Subsequent research has further investigated the shape, slope, and index of the acromion, along with its coverage, to consider their potential effects on RC degeneration [26-28].
INTRINSIC FACTORS
The intricacies of RCT healing span a myriad of factors, both intrinsic and extrinsic, that together orchestrate this multifaceted process. In the absence of RCT, the subacromial space may be compromised by several factors, such as a tight posterior glenohumeral capsule, aberrant scapular muscle activity, scapular dyskinesis, thoracic hyperkyphosis, and acromioclavicular joint arthritis. In a study of 50 pairs of elderly twins aged between 50 to 75 years, it was determined that anatomical features that influence the width of the subacromial space are predominantly genetically determined, with minimal influence from external factors. These genetic factors also play a critical role in determining the acromiohumeral distance [29]. In addition, arthroscopic findings often reveal that degeneration and tear of the cuff begin on the joint side, significantly undermining the potential role of subacromial influences. Moreover, lifestyle choices and habits, such as smoking, high body mass index, uncontrolled high blood pressure, and alcohol consumption, further compound these intrinsic influences [30-33].
Regarding the implications of hyperlipidemia and diabetes, studies have shown that elevated cholesterol levels significantly impact the RC healing process. A rat model study revealed that hyperlipidemia's effects persist despite surgical repair, likely affecting the tendon's healing potential [34]. Patients with hyperlipidemia are 6.5 times more likely to experience retears than those with normal lipid levels [35]. The combination of an anteroposterior tear size of over 40 mm, hyperlipidemia, and critical shoulder angle exceeding 37° dramatically increases the prediction probability for retear to 86.2% [36]. Moreover, the failure rate of RCT healing was found to be significantly higher in diabetic patients compared to non-diabetic ones [37].
All these intrinsic factors have tissue hypoxia in common, induced by alterations of the peripheral microcirculation, which appears to be a critical link. The cuff draws its vascular supply from the myotendinous junction, supplemented by osteo-tendon vascularization and bursal side vascularization. Without this blood supply, the cuff degenerates and fails to heal. Hypoxia is an essential factor in tendon degeneration as it triggers the formation of reactive oxygen species (ROS), leading to apoptosis and a shift in cellular metabolism towards glycolysis [38]. These changes result in the production of intermediates such as lactic acid and succinate, which in turn enhance the secretion of interleukin (IL)-6, IL-23, and IL-1β. Hypoxia also causes a downregulation of type I collagen genes and an increase in type III collagen genes in tenocytes, disrupting collagen I homeostasis. Conditions such as hypercholesterolemia, diabetes, and obesity exacerbate these processes, thereby accelerating tendon senescence [39]. An accumulation of ROS induces tenocyte apoptosis through the action of mediators like metalloproteinases, cytokines, and Jun N-kinase, which, when phosphorylated, lead to cell death. Over time, apoptosis results in matrix degeneration, reduced collagen synthesis, decreased mechanical tendon resistance, and consequently, tissue degeneration and rupture.
To further comprehend these processes, patients with RCTs were subjected to capillaroscopic examination to analyze the peripheral microcirculation of the upper limbs. The study’s focus was particularly on the presence of dilated capillaries, capillary morphology, hemosiderin deposits, visibility of the subpapillary venous plexus, pericapillary edema, capillary blood flow, ectasias, microaneurysms, and neoangiogenesis. The findings revealed a significant association between these variables and the presence of a RCT, supporting the hypothesis that microcirculation disorder plays a vital role in cuff degeneration and subsequent tendon rupture [40].
Despite the plethora of intrinsic and extrinsic factors contributing to RC degeneration and tear, it's noteworthy that a study conducted on monozygotic and dizygotic twins over 60 years of age revealed that only 18% of the genesis of the cuff degeneration and tear could be attributed to genetic factors. Shared environmental factors and unique environmental factors, such as working, sports activity, comorbidities and poor habits, play a more significant role in influencing RC degeneration than genetic factors. Collectively, these factors account for more than 80% of the total heritability index. [41].
TENDON HEALING AFTER REPAIR
The journey through RCT healing is complex, involving a remarkable interplay of proteins, cells, and healing techniques that bring together intricate biological processes. Exploring this topic, the role of periostin, a matricellular protein highly implicated in fracture healing, is being considered in the process of tendon repair. Correlations have been demonstrated between periostin synthesis and the healing process of flexor digitorum longus tendon graft in a mouse model, suggesting its importance in tissue repair. Specifically, the splice variant of periostin interacts with the extracellular matrix, inciting myofibroblast migration. Myofibroblast cells have a critical role in type I collagen production during tissue repair [42]. Interestingly, in the context of a RCT, there is a high level of immunohistochemical expression of periostin at the tear margins, while it's scarcely expressed in healthy tendons [43]. This discrepancy indicates a potential role of periostin in RC degeneration and tear, and, in broader terms, in tendon healing mechanisms.
Moreover, the role of nuclear factor-kB (NF-kB), a key transcription factor in the immune system, cannot be overlooked in the evolution of RCT and the healing mechanism of cuff tendons. This regulatory protein modulates the expression of crucial biomolecules like inducible nitric oxide synthase, cytokines, cyclooxygenase, growth factors, and effector enzymes. Interestingly, NF-kB activation is observed in RCTs but is relatively low in healthy tendons. This activation might result from tissue apoptosis or hypoxia, further highlighting the complex interplay of factors during the tendon healing process [44].
Understanding tendon healing after repair necessitates a deeper look into surgical techniques, particularly those that might induce further ischemia, such as the double-row or suture bridge technique [45]. This highlights the need for refining and developing surgical interventions that can minimize further damage and promote optimal healing environments for torn tendons.
Nuclear lamin A, an anti-apoptotic and cell mechanostat factor located in the nuclear envelope, is a crucial player in maintaining cellular stability. Lamin A, which provides an anchoring site for nuclear pore complexes, is believed to offer protection against apoptosis and mechanical insults, thereby preserving nuclear integrity. Immunohistochemical investigations reveal its functionality in cells of healthy tissues or cells near the margins of either small or large lesions. However, lamin A's protective action appears to be lost in the cells of massive tears, emphasizing the importance of early repair of small RCTs. The high retear rate in massive cuff repairs might be attributable to cellular apoptosis and nuclear modifications instigated by a lack of lamin A [46].
As the intricacies of RC repair are traversed, a vital question confronts us: can we tilt the scales to favor healing? The path to avoiding repair failure must lie in a technique that ensures robust fixation but primarily hinges on facilitating the natural healing process. Several methods, such as platelet-rich plasma therapy, microfracture, acromioplasty, and the use of autologous microfragmented lipoaspirate tissue, are showing promise in this respect [47-49].
In research on the origin of tendon healing cells, multiple animal studies point to an unexpected source: the paratenon. Traditionally, the belief was that tendon healing cells originate from the bone or the tendon, but this view is shifting. It is now proposed that the so-called 'bursa' is, in fact, the paratenon of the RC, and that this bursa contains the cells necessary for healing [50]. This hypothesis underscores a critical shift in understanding RC healing, paving the way for new strategies in promoting optimal tendon recovery and minimizing the risk of retear.
CONCLUSIONS
Biological factors may contribute positively to the healing process, irrespective of the size of the tear. However, a word of caution is expressed against certain repair techniques that potentially favor ischemia, as they are assessed not to facilitate healing. As the exploration into this subject of RC tear healing continues, the aim should be to fine-tune understanding and strategies to optimize patient outcomes. It is the hopeful expectation of the authors that this review will stimulate further investigation into the complex dynamics of RC degeneration and healing.
Notes
Author contributions
Conceptualization: SG, HK, HSS. Data curation: SG, HK, YJ. Formal analysis: HK, YJ. Investigation: HK, YJ. Methodology: SG, HK, YJ, HSS. Resources: SG. Writing – original draft: HK. Writing – review & editing: SG, HSS.
Conflict of interest
None.
Funding
None.
Data availability
None.
Acknowledgments
None.