Postoperative pain control for shoulder arthroplasty
Article information
Abstract
Since its inception in 1893, shoulder arthroplasty has become an increasingly common surgical procedure. Between 1998 and 2008, shoulder arthroplasty procedures increased by nearly 28,000 cases per year in the United States alone and is the fastest growing joint replacement surgery among all joint. Despite its advantages, shoulder arthroplasty is often accompanied by significant postoperative pain. Pain control continues to be a major concern in patient management, as it impacts operative costs, postoperative mobility, length of hospital stay, patient satisfaction, and overall surgical outcomes. This review aims to provide an overview of drugs such as opioids and regional anesthetics, as well as methods such as local wound infiltration, nerve block, brachial plexus infiltration, cryotherapy and multimodal approaches employed in postoperative shoulder arthroplasty pain control.
INTRODUCTION
Since its inception in 1893, the shoulder arthroplasty procedure has become increasingly common [1-3]. Between 1998 and 2008, shoulder arthroplasty procedures increased by nearly 28,000 cases per year in the United States alone, and it is the joint replacement surgery with the fastest rise in number of procedures performed annually [1-3]. The evolving prostheses, techniques, and instruments have facilitated treatment utility for various shoulder pathologies, including a variety of glenohumeral arthritides, fractures, osteonecrosis, and posttraumatic sequelae [1,2].
Common types of shoulder arthroplasty include anatomic total shoulder arthroplasty (TSA) and reverse TSA [4]. A 2023 article reported trends in anatomic TSA and reverse TSA analyzed via a multi-country registry. While use of reverse TSA is increasing at a rate greater than that of anatomic TSA, it may be for good reason. Reverse TSA demonstrates lower 8-year revision rates, rotator cuff tears and subscapularis failure [4].
Despite their advantages, most forms of shoulder arthroplasty are often accompanied by significant postoperative pain [5,6]. Pain control continues to be a major concern in patient management, as it impacts operative costs, postoperative mobility, length of hospital stay, patient satisfaction, and overall surgical outcomes [7,8]. An additional consideration in postoperative pain management has been the use and misuse of opioid analgesics. Despite effectiveness in pain control, opioids have inherent addictive risks. Past literature indicating the over-prescription of opioids in upper extremity surgery has highlighted the need for more responsible opioid stewardship in discussions regarding surgical analgesia [9]. This review aims to provide an overview of drugs and methods of utilization currently employed in postoperative shoulder arthroplasty pain control.
ORAL ANALGESIA (DRUGS AND REGIMES)
Opioids
While opioids effectively improve postoperative pain, over-prescription and addiction are recognized concerns, amongst other side effects [7,8,10]. The 2015 National Survey on Drug Use and Health estimated 91.8 million U.S. adults consumed prescription opioids, of whom 11.5 million were estimated to have misused opioids, with 1.9 million subsequently developing a use disorder [10].
In addition to increased physical dependence and mortality, postoperative opioid use is associated with several short- and long-term effects. These effects include secondary hyperalgesia, constipation, respiratory depression, nausea, vomiting, pruritus, drowsiness, confusion, and coma [7,8]. Recipients of shoulder surgery, especially arthroplasty, may receive opioid prescriptions that far exceed the guidance of the Prescribing Opioids for Postoperative Pain–Supplemental Guidance developed by Dr. Robert Bree Collaborative and Washington State Agency Medical Directors’ Group. This guidance suggests 42 opioid tablets for a medium term recovery operation [7,11].
It should be noted that the side effects of opioid consumption may spread beyond the individual receiving opioid prescription to others who are not recipients of the prescription as excess opioids may become available to second party individuals. This is of specific concern for the orthopedic community, which prescribes an average of 32 unused opioid tablets following shoulder operations [12].
Acetaminophen
Acetaminophen is often used in relief of mild to moderate pain, and is commonly used in multimodal analgesia approaches [13]. In the orthopedic setting, including shoulder arthroplasty, acetaminophen has demonstrated efficacy in pain reduction [7,13]. Intravenous (IV) acetaminophen, while effective, lacks superiority compared to oral acetaminophen [13]. In a study comparing oral acetaminophen (2×500-mg capsules) given preoperatively to IV acetaminophen (1,000 mg/100 mL) perioperatively in hip and knee arthroplasty, no significant differences were noted in the 24 hours following surgery [13]. Support for the effectiveness of acetaminophen in the postoperative period is not robust and requires further study [7].
Nonsteroidal Anti-inflammatory Drugs
To our knowledge, no study has directly analyzed the impact of nonsteroidal anti-inflammatory drugs (NSAIDs) on shoulder arthroplasty outcomes with regard to pain control, hospital stay, and postoperative opioid consumption [14]. However, NSAIDs (flurbiprofen) have been demonstrated to decrease visual analog scale (VAS) at 6 hours post-surgery, opioid consumption 2 hours post-surgery, and to increase time before additional analgesic requirement in shoulder arthroscopy [14,15]. Despite their analgesic utility, further evaluation of NSAIDs for shoulder arthroplasty is necessary, especially in the context of bone metabolic changes and tissue repair [14].
Gabapentin
In the setting of shoulder arthroplasty, preoperative gabapentin has been shown to lessen opioid use and therefore opioid side effect burden compared to individuals who did not consume gabapentin preoperatively. Additionally, patients who received gabapentin preoperatively were reported to have less chronic pain following their procedures [16].
REGIONAL ANALGESIA
Pharmaceutical Agents
Lidocaine, bupivacaine, and ropivacaine
Lidocaine, bupivacaine, and ropivacaine are anesthetics commonly used as nerve block analgesics. A study directly comparing 1% lidocaine to 0.2% ropivacaine in perioperative interscalene nerve blocks demonstrated that lidocaine had a significantly faster time of onset compared to ropivacaine (lidocaine 7.5 minutes vs. ropivacaine 30 minutes). Despite lidocaine’s faster action, its anesthetic effect was not superior. Within the first 8 hours of infusion, patients receiving lidocaine required significantly more rescue IV tramadol. Between postoperative hours 16 and 24, a greater regression in motor nerve blockade was recorded in the ropivacaine cohort, demonstrating enhanced motor function in the ropivacaine group compared to the lidocaine group (Table 1) [17].
To compensate for lidocaine's shorter duration of action it is often combined with bupivacaine or ropivacaine for peripheral nerve blocks [17,18]. Although comparisons of bupivacaine and ropivacaine alone have yielded comparable efficacy, this does not remain true in the presence of lidocaine. Nishiyama et al. [18] compared efficacies of interscalene blocks of 15 mL of ropivacaine 0.375%, ropivacaine 0.75%, bupivacaine 0.25%, or bupivacaine 0.5%, all combined with lidocaine 1.0 % 15 mL. Both ropivacaine groups had slower motor block initiation, and longer sensory and motor block durations than the bupivacaine groups.
Liposomal bupivacaine
In 2011, liposomal bupivacaine (Exparel) was approved by the U.S. Food and Drug Administration [2,19]. Liposomal bupivacaine utilizes the release of bupivacaine hydrochloride from lipid bi-layers and the subsequent binding of voltage-gated sodium channels prevents nerve depolarization [2,19,20]. The most notable attribute of this technique arises from its mechanism, which allows analgesic release over 72–96 hours post-injection. This characteristic directly contrasts the limitation of short duration time in non-liposomal bupivacaine peripheral block approaches and local anesthetic infiltrations that do not utilize a postoperative conduit of administration [2,20].
Since its approval, local infiltration analgesia (LIA) has been the most common use of liposomal bupivacaine [19]. In comparison to LIA with traditional bupivacaine, LIA with liposomal bupivacaine has demonstrated significant benefits, including pain reduction and decreased length of hospital stay [21]. The benefits of liposomal bupivacaine-LIA compared to single-injection interscalene nerve block are most noticeable after 8 hours, with a lesser effect between 0-8 hours [2,22-24]. Length of hospital stay, opioid consumption, and postoperative pain were compared between patients receiving either liposomal bupivacaine (LB) through pericapsular and wound layer areas or single-shot interscalene nerve block (ISNB) of 30 mL of 5% ropivacaine following shoulder arthroplasty were analyzed by Hannan et al. [2]. Between 0–1 hour and 8–14 hours, no significant difference in pain numerical rating scale (NRS) was reported between the liposomal bupivacaine and ISNB groups. However, at time intervals of 18–24 and 27–36 hours, there was a significantly lower mean NRS score for patients being treated with liposomal bupivacaine [2]. Opioid consumption was found to be significantly greater for the ISNB group on postoperative days (PODs) 2 and 3 than for the liposomal bupivacaine group. On the day of surgery, 43% of liposomal bupivacaine patients were released from the hospital compared to less than 10% of ISNB patients. Mean hospital stay was also shorter for the liposomal bupivacaine cohort compared to the ISNB cohort [2]. A reduced short term effect with greater long term analgesia of LB was corroborated by a prospective randomized trial which demonstrated a decrease in morphine use 13–16 hours postoperation, but significantly more pain within the first 8 hours [23]. Furthermore, a meta-analysis reported no significant postoperative VAS pain score difference between LB and ISNB within the first 8 hours, but an increase at both 12 and 24 hours for those treated with ISNB [24].
Liposomal bupivacaine via LIA (LB-LIA) can avoid the side effects of ISNB, especially of motor block and injury of peripheral nerves, while taking advantage of the decreased complexity and side effect profile of LIA administration [23]. However, LB-LIA does have a side effect profile, most notably of nausea, vomiting, constipation, pyrexia, dizziness, and headaches [2,24,25]. Although local infiltration has become the most common use of liposomal bupivacaine, its use in other techniques is expanding [2,19]. Field block, or analgesic infiltration encircling the surgical site, has demonstrated advantages over ISNB in pain reduction and reduced opioid consumption, and may be beneficial in instances of wound contamination and abscesses [7,26].
Liposomal bupivacaine use in ISNB is a newer practice. It has demonstrated effectiveness in areas including analgesia and decreased opioid consumption [3,27]. Despite this, risks of liposomal bupivacaine in ISNB are apparent. In a study of 352 shoulder surgery patients that received an interscalene block using liposomal bupivacaine, 10.5% had minor pain control complications, but 6% of patients presented to the emergency department due to major complications. The most common complication was chest pain and shortness of breath [27]. The cardiopulmonary symptoms were thought to be associated with the interscalene block mechanism, and not the liposomal bupivacaine. ISNB’s intended function is to inhibit C5-C7, but C3 and C4, the phrenic nerve controlling the hemi-diaphragm, is periodically affected. Diminished hemi-diaphragmatic activity impairs breathing and increases the need for cardiac output. The American Society of Anesthesiologists’ score, age, and Charlson Comorbidity Index were all found to be significant predictors of postoperative complications and should be considered in all instances of liposomal bupivacaine in ISNB [27]. Additionally, patients with pre-existing cardiopulmonary disease who are contraindicated for ISNBs may benefit from multimodal pain management using LIA or field block with liposomal bupivacaine.
Techniques
Local anesthetic infiltration in the wound
In multimodal approaches, infiltration at the wound has proved beneficial over controls that use no local infiltration [28]. However, outcomes for the use of LIA as an alternative to regional nerve blocks are more complex [5-7,28,29]. In regard to regional anesthetic blocks, such as single-shot ISNB, the literature has provided conflicting information on the efficacy of LIA immediately following surgery, but suggests that LIA is a superior analgesic up to 96 hours postoperatively [2,5,6,23,28]. A prospective, randomized, comparative non-inferiority study comparing ISNB (20 mL of 0.2% ropivacaine) and LIA (110 mL of 0.2% ropivacaine with 30 mg of ketoprofen and 0.5 mg of epinephrine) demonstrated significantly more pain among ISNB patients in the recovery room, significantly more morphine use in the recovery room, and significantly more movement-associated-pain on day 2 following surgery [6]. Similar studies using LIA with extended release analgesics (liposomal bupivacaine) have also shown advantages to LIA over ISNB in regards to comparable pain levels following the operation, enhanced pain management 18–24 hours postoperatively, and decreased opioid consumption [2,23].
A notable benefit of anesthetic infiltration in-situ is the absence of complications often presenting with peripheral nerve blocks. LIA can be administered without resulting rebound pain, paresthesia, chronic nerve damage, or muscle weakness [6]. This advantage is further emphasized when comparing LIA to methods employing catheterization. In the article by Bjørnholdt et al. [5], continuous interscalene block (CISB) was proven superior to LIA in pain management, but two patients undergoing CISB experienced considerable complications. The first patient acquired severe dyspnea, a pulmonary embolism 8 days postoperatively, and phrenic nerve palsy lasting >3 months. The second patient had a 2-month duration of “pin prick sensation” in the forearm and thumb. No complications of this degree were noted in the LIA cohort [5]. Additionally, unlike nerve blocks that require technical equipment and a senior anesthesiologist, local infiltration has a simpler administration protocol that can be performed by the surgeon [2]. The safety and straightforward administration of this method makes LIA a point of interest in multimodal analgesia approaches.
Interscalene nerve block
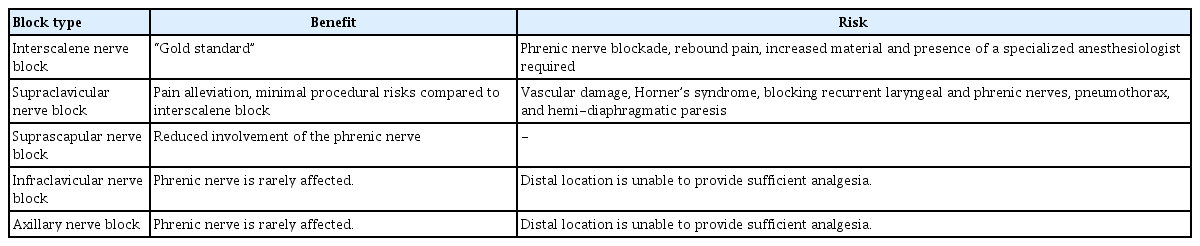
ISNB is a regional anesthetic administered at the C5 root, C6 root, and superior trunk of the brachial plexus which allows analgesia at the lateral two-thirds of the clavicle, the proximal humerus, and the glenohumeral joint [8]. This technique is the most common analgesic method used in shoulder arthroplasty and has been viewed as the “gold standard” of pain treatment due to its effectiveness in postoperative pain management up to 4-8 hours, overall patient satisfaction, decreased need for opioids, and patient rehabilitation outcomes [2,8,30,31]. This technique is performed by using anatomic landmarks, nerve stimulation, or ultrasound guidance, all of which are considered indirect visualization methods. Despite efficacy of ISNB side effects exist. Ipsilateral blockade of the phrenic nerve has led to significant cardiopulmonary complications, recurrent laryngeal nerve palsy, persistent neurological problems, Horner’s syndrome, pneumothorax, cardiac arrhythmia, hoarseness, and failure to act on the lower part of the brachial plexus have all been reported. Rebound pain is another common side effect of ISNB [2,8,14,27,30]. Once the analgesic effects of the ISNB dissipate, an acute pain sensation can occur, most commonly between 8–24 hours postoperatively. This pain spike often accompanies increased opioid consumption. Furthermore, the complexities of this procedure necessitate more equipment, higher cost, lengthened procedure time, and presence of a specialized anesthesiologist [2,8,14,27,30].
Continuous interscalene block
The effects of ISNB can be extended up to 48 hours with the use of a perineural catheter (CISB) that allows analgesic administration after the initial interscalene block [7,30]. This technique has exhibited a greater efficacy over traditional ISNB, which has a limited duration of effect [24].
Various studies have demonstrated the efficacy of CISB in reducing pain, opioid consumption, and length of hospital stay [7,8]. Not only has CISB demonstrated its benefit over single shot ISNB, but it has also proven superior in efficacy compared to multimodal analgesic methods such as ISNB and LIA. A retrospective review comparing 53 patients receiving ISNB with local infiltration analgesics and 63 patients receiving CISB for treatment of shoulder arthroplasty demonstrated a significant increase in the need for opioids in the cohort receiving ISNB with LIA on POD 0 and 1. On POD 1, the CISB reported significantly decreased pain [32].
Despite its advantages, CISB have many acquired risks. Mobilization of the catheter, administration difficulty, permanent neurological risks, high costs, increased need for clinical care, and further medical complications are barriers to perineural catheterization [6,30,32]. CISB has consistently demonstrated efficacy over LIA due to its magnitude and duration of pain control [5,25,29]. A randomized clinical trial of 125 patients receiving CISB, single-shot ISNB, and LIA demonstrated a significantly improved Overall Benefit of Analgesia Score, lower pain score, and lower opioid consumption in the CISB cohort compared to the latter two techniques [29]. Bjørnholdt et al. [5] compared local anesthetic infiltration (150 mL ropivacaine 0.2 % with epinephrine intraoperatively) to CISB (ropivacaine 0.75 %, 7 mL bolus followed by 48-hour 5 mL/hr infusion via catheter) and demonstrated similar results [5,29]. Within the first 8 hours, pain scores for the group receiving local ropivacaine were significantly higher. Opioid use within 24 hours of shoulder replacement was 95 mg in the local infiltration group and 40 mg in the CISB group (P=0.0001). In a study contrasting liposomal bupivacaine to indwelling interscalene nerve block (IINB), results were consistent with the previous findings, despite liposomal bupivacaine’s ability to extend release similar to that of a catheter [25]. In this study, 46 patients received indwelling inter scalene nerve block (IIBN; control) and 37 received LB via local infiltration. In the post-anesthesia care unit, average highest recorded VAS scores (0–10) were 7.25 for the LIA cohort and 1.91 for the IINB cohort (P<0.005). VAS score was significantly higher in the LIA-liposomal bupivacaine cohort as opposed to the IIBN cohort for the duration of POD 0. The remainder of hospitalization days did not render any significant difference in VAS score [25]. Post-anesthesia care unit opioid use was found to be significantly higher for the LB group as opposed to the control. This trend continued throughout the duration of hospitalization with mean total opiate use for the LIA group being more than two times that of the control group [25].
Supraclavicular nerve block
The supraclavicular nerve stems from superficial cervical plexus C3/C4 nerve roots [33]. Supraclavicular nerve blocks involve analgesic introduction between the anterior and middle scalene muscles to target and anaesthetize the supra-clavicular nerves. The procedure can be performed by clinical localization or under ultrasound visualization. This technique has been successful in pain alleviation, but significant side effects have been reported [7,8,31,34,35]. Vascular damage, Horner’s syndrome, blocking recurrent laryngeal and phrenic nerves, pneumothorax, and hemi-diaphragmatic paresis are all reported in the literature [8,31,34,35]. The use of ultrasound to ensure nerve localization and precise needle placement for a supraclavicular block has been proven to reduce some procedure-associated risks, including pneumothorax [36].
Despite side effects, a supraclavicular block may minimize procedural risks compared to the commonly used interscalene block while providing effective analgesia [34-37]. Risk of nerve injury, hemi-diaphragmatic paresis, and Horner’s syndrome have been significantly lowered in patients receiving the supraclavicular block [34-36]. Comparable procedure times and comparable pain scores have been reported 24 hours postoperation among the two techniques [34-36]. When compared to interscalene blocks, multimodal pain control approaches incorporating a supraclavicular nerve block yield comparable pain scores and opioid consumption post-surgery, while avoiding the higher risk of adverse events associated with ISNB [37].
SUPRASCAPULAR BLOCK: AN ANATOMICALLY SUPERIOR DISTAL APPROACH
Suprascapular blocks are performed more distal than interscalene or supraclavicular blocks, thereby avoiding phrenic nerve involvement, allowing for reduction of pulmonary complications. Their location also decreases symptoms of Horner’s syndrome, dyspnea, and hoarseness [38].
INFRACLAVICULAR AND AXILLARY NERVE BLOCK: AN ANATOMICALLY INFERIOR DISTAL APPROACH
Distal nerve block approaches, including infraclavicular and axillary nerve blocks, may be considered specifically in patients with respiratory impairment, since the phrenic nerve is rarely affected [38]. However, the distal location is unable to provide appropriate analgesia in shoulder arthroplasty when used in isolation. In combination with blocks such as the suprascapular that are administered more laterally with respect to the phrenic nerve, appropriate and safe analgesia can be achieved by flanking the glenohumeral joint [38-41].
Axillary nerve blocks, used most commonly for distal upper limb analgesia, have demonstrated use in pain reduction in selected cases [39,40,42]. Risks such as nerve injury, vascular injury, and unsuccessful block delivery, with subsequent impact on increased costs, time, and supplies utilized, have led to techniques to reduce such outcomes [42,43]. Associated ultrasound guidance, paresthesia technique, trans-arterial technique, and peripheral nerve stimulation have all fostered increased success rates and decreased adverse events [42,43].
The variety of nerve blocks described above are listed in Table 2 [2,7,8,30,31,34,35,38-43]. Although these techniques have demonstrated independent efficacy in dealing with various types of upper extremity surgery, their use in conjunction with techniques such as suprascapular nerve block have proven safe and effective in shoulder surgery [39-42]. The combined utility of distal nerve blocks coupled with suprascapular blocks is discussed further in the multimodal approaches section.
Open Local Infiltration around the Brachial Plexus
Various anesthetic adjuvants have been studied for their efficacy in the local infiltration around the brachial plexus. In a randomized, double-blind, placebo-controlled study, following shoulder surgery, dexamethasone improved block duration and postoperative opioid consumption was significantly lower. Study groups with dexamethasone adjuvant had a significant decrease in antiemetic use and no significant differences in patient satisfaction [44]. The article by Kamineni and Cheppalli [45] describes significant pain control, by placing local anesthetic, in liquid and slow release forms, under direct visualization around the brachial plexus, using the same surgical approach as the open shoulder surgery. In this approach the senior author describes a satisfactory outcome rate of 96% of the more than 200 patients who underwent brachial plexus block for shoulder arthroplasty over 5 years [45].
The mean surrounding volume of local anesthesia required for each nerve to have an effective blockade has also been clinically studied. The radial nerve required 3.42 mL, while 2.75 mL, 2.58 mL, and 2.30 mL were the requirements for the median, ulnar, and musculocutaneous nerves, respectively. During this study, seven of the nineteen participants needed more local anesthesia than the initial dose, and two required additional IV boluses of fentanyl [46].
MULTIMODAL APPROACHES
The combination of various analgesia techniques may allow an optimized pain control protocol following shoulder arthroplasty. Discussion on the additive benefits of local infiltration, interscalene block, supraclavicular block, as well as similar techniques, is not abundant in the literature [7]. However, the existing literature does show utility in a multimodal mechanism [12,14,37].
Liposomal Bupivacaine Combined with Suprascapular Nerve Block
One study examining the efficacy of multimodal pain control protocols compared ISNB with bupivacaine to the combined effect of liposomal bupivacaine field block, suprascapular nerve block, and ISNB with bupivacaine. In this study, 50 patients undergoing rotator cuff repair were divided into two groups. The control group received an interscalene block of 0.5% bupivacaine. The treatment group received both a liposomal bupivacaine field block and suprascapular nerve block in conjunction with the control ISNB. Study outcomes demonstrated significantly reduced pain scores among the treatment group for the first 48 hours following the operation. Additionally, a 64% decrease in postoperative narcotic use was reported for the treatment group [12]. A similar study comparing interscalene block in the presence or absence of local infiltration of liposomal bupivacaine demonstrated no significant difference in pain scores up to 72 hours postoperatively, but reported significantly less narcotic consumption among the group receiving interscalene block without local infiltration of liposomal bupivacaine [9].
Supraclavicular Nerve Block with Suprascapular Nerve Block
Information regarding the efficacy of supraclavicular blocks compared to that of interscalene blocks is generally lacking [35]. However, when used in combination with a suprascapular block, supraclavicular blocks are able to demonstrate comparable analgesia to ISNB. In a study by Trabelsi et al. [37], patients were divided into groups receiving either an ultrasound guided supraclavicular block (15 mL of bupivacaine 0.25%) combined with an ultrasound guided suprascapular block (15 mL of bupivacaine 0.25%) or an ultrasound guided interscalene block (30 mL of bupivacaine 0.25%). The amount of time required for both motor and sensory blockage was not significantly different. Furthermore, no significant difference in pain score or opioid consumption was demonstrated in the first 24 hours postoperation. The suprascapular block/supraclavicular block procedure took significantly longer to administer, and although no impediments were noted, it had no safety concerns beyond those of an interscalene block. Phrenic nerve blockade was reported in all patients receiving ISNB [37].
Axillary Nerve Block Combined with Suprascapular Nerve Block
Combining axillary and suprascapular nerve blocks has proven to be a successful postoperative pain strategy [39,40,47]. A study of 20 patients receiving a combination of suprascapular and axillary nerve blocks for shoulder arthroscopy showed that no additional perioperative analgesics, including opiates, or general anesthesia, were needed. Mild to moderate pain in 15 patients was treated with NSAIDs and did not require opioid treatment. 100% of patients reported they would re-undergo this technique [39].
Studies comparing the combination of suprascapular nerve block and axillary nerve block to interscalene nerve block have been reported. Patient satisfaction, complication rate, and negative side effects were equivalent among both groups. Mean length of analgesia provided in the combination cohort was 5.9 hours longer than that of the interscalene group (P=0.002). Despite this, immediate postoperative opioid consumption among the combination cohort was significantly higher. However, at 24 hours post-surgery, no significant difference in morphine consumption was reported [47]. Although effective in reducing phrenic block risk, an analgesia approach involving axillary nerve block combined with suprascapular nerve block still exhibits the absence of blockade to some sensory components of the shoulder [41].
Infraclavicular Brachial Plexus Block Combined with Suprascapular Nerve Block
In infraclavicular-suprascapular combined blocks, the infraclavicular block can target brachial plexus cords, while the suprascapular block anesthetizes the remainder of the shoulder. In a study of patients undergoing combined infraclavicular (15 mL of ropivacaine 0.75%) and suprascapular blocks (4 mL of ropivacaine 0.5%) for shoulder arthroplasty, effective analgesia and decreased hemi-diaphragmatic paralysis risk was demonstrated. On average, the combined block took 7.2 minutes to administer, and median time of pain relief was 12.7 hours. Postoperative hours 1, 3, 6, 8 and 24 corresponded with median NRS values 1, 0, 0, 0 and 3. Median use of 52.5 mg of oral morphine equivalent was reported in the first postoperative 24 hours. No patients were noted to display dysphagia, dyspnea, hoarseness, Horner’s syndrome, or pneumothorax. A single patient (5%) experienced hemi-diaphragmatic paralysis. Musso et al. [48] reported no interscalene block technique resulted in a risk of hemi-diaphragmatic paralysis less than 27%.
Periarticular Injection Combined with Nerve Block
Although primarily reported on in the knee joint, periarticular injection in combination with nerve block has been demonstrated to be a successful analgesic in the instance of shoulder arthroplasty. Vijittrakarnrung et al. [49] describe the benefit of using a periarticular injection (comprised of ropivacaine, epinephrine, clonidine, and ketorolac) with an interscalene nerve block compared to an interscalene nerve block alone. Periarticular injection combined with interscalene block led to significantly less postoperative opioid use and VAS pain score within the first postoperative day compared to only interscalene block [49].
CONCLUSIONS
Although interscalene nerve blocks have been viewed as the “gold standard” in minimizing pain and decreasing opioid consumption, the procedure is not without risk [2,8,30]. New delivery and formulations may provide safer and effective alternatives for pain management.
Notes
Author contributions
Conceptualization: MW, SK. Project administration: MW, SK. Supervision: SK. Validation: SK. Writing – original draft: MW. Writing – review & editing: SK.
Conflict of interest
None.
Funding
None.
Data availability
Contact the corresponding author for data availability.
Acknowledgments
None.