Outcomes of arthroscopic capsulolabral reconstruction for anterior instability with greater than 20% glenoid bone defects: are Latarjet procedures absolutely indicated for these patients?
Article information
Abstract
Background
Recent studies have reported high rates of recurrence of shoulder instability in patients with glenoid bone defects greater than 20% after capsulolabral reconstruction. The purpose of the present study was to evaluate the failure rate of arthroscopic capsulolabral reconstruction for the treatment of anterior instability in the presence of glenoid bone deficits >20%.
Methods
Retrospective analyses were conducted among cases with anterior shoulder instability and glenoid bone defects of >20% that were treated by arthroscopic capsulolabral reconstruction with a minimum 2-year follow-up (30 cases). We included the following variables: age, bone defect size, instability severity index score (ISIS), on-/off-track assessment, incidence recurrent instability, and return to sports.
Results
The mean glenoid bone defect size was 25.8%±4.2% (range, 20.4%–37.2%), and 18 cases (60%) had defects of >25%. Bony Bankart lesions were identified in 11 cases (36.7%). Eleven cases (36.7%) had ISIS scores >6 points and 21 cases (70%) had off-track lesions. No cases of recurrent instability were identified over a mean follow-up of 39.9 months (range, 24–86 months), but a sense of subluxation was reported by three patients. Return to sports at the preinjury level was possible in 24 cases (80%), and the average satisfaction rating was 92%.
Conclusions
Arthroscopic soft tissue reconstruction was successful for treating anterior shoulder instability among patients with glenoid bone defects >20%, even enabling return to sports. Future studies should focus on determining the range of bone defect sizes that can be successfully managed by soft tissue repair.
INTRODUCTION
Burkhart and De Beer [1] reported that treatment of glenohumeral joint instabilities with significant defects of the glenoid cavity by arthroscopic capsulolabral reconstruction alone had a failure rate of 67%. Thus, it is generally accepted that glenoid bone defects >20% cannot be overcome by treatment limited to arthroscopic soft tissue procedures [2-5]. More recently, the glenoid track concept was developed while considering the combined effects of glenoid bone defects and bone defects of the humeral head (Hill-Sachs lesion) on anterior shoulder stability [6]. According to the glenoid track concept, procedure such as the Latarjet procedure are recommended for treating off-track cases, in which the glenoid and humeral head bone defects are sufficient to allow the humeral head to engage on the glenoid rim [7].
In our practice, arthroscopic capsulolabral reconstruction has remained the first-line approach for the treatment of anterior shoulder instability with glenoid bone loss in the range of 20%–30%, after careful discussions about surgical options with patients. Therefore, the purpose of the present study is to document the failure rate of arthroscopic soft tissue reconstruction for the treatment of anterior shoulder instability in patients with glenoid bone deficits >20%. We hypothesized that arthroscopic anterior soft tissue stabilization would yield clinically satisfactory outcomes with a low recurrence rate.
METHODS
This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. J-1705-082-855) and Seoul National University Bundang Hospital (IRB No. B-1706-402-102).
Study Design and Criteria
The study was conducted using a retrospective case series design. Between January 2004 and December 2015, 372 patients (261 and 111 at two institutions) underwent arthroscopic anterior capsulolabral reconstruction with or without other arthroscopic soft tissue procedures such as concomitant superior labral anterior and posterior (SLAP) repair, the remplissage procedure, and/or interval closure for anterior shoulder instability. The study group was selected by applying the following criteria: (1) glenoid bone defect >20%, calculated on preoperative computed tomography (CT) or magnetic resonance imaging (MRI) (37/372 cases), and (2) availability of follow-up data for at least 2 years postoperatively. Patients that underwent a bony procedure (e.g., Latarjet or free-bone graft), revision, concomitant rotator cuff repair, and patients with multi-directional instability or epileptic dislocation were excluded (34/372 cases). Of the 372 cases, 34 (9.9%) met our inclusion criteria. However, of these 34 cases, two were revision cases and two underwent the Latarjet procedure, and thus, these four patients were excluded. Accordingly, 30 cases constituted the study cohort.
Information about hand dominance, age at onset of shoulder instability, number of dislocations, subjective ease of dislocation, possibility of self-reduction, level of sports activity, and occupation were extracted from medical records, along with basic demographic data. Level of sport activity was dichotomized as competitive or recreational based on requirement of primary occupations. Assessments of hyperlaxity (external rotation >85° or hyperabduction of ≥20° between sides) [7] were included in the physical examination.
The definition of what constitutes a large or significant amount of bone loss is controversial, but based on recent consensus guidelines, a large defect is defined as 20%–30% loss of glenoid width and <21% loss of glenoid length [2-4]. Therefore, we set the standard at 20%. The prevalence of glenoid bone defects >20% among 372 patients that underwent arthroscopic anterior capsulolabral reconstruction was 9.9% (37/372 cases), which is lower than a previously reported rate of 12% [8]. We cautiously hypothesized that transfer bias would not be high among 9.0% of cases (3/33) lost to follow-up. The study protocol and data collection were approved by our institutional review board (IRB No. B-1706-402-102, J-1705-082-855).
Surgical Procedures and Rehabilitation
All surgical procedures were performed by two surgeons (JHO and SHK). All arthroscopic procedures were performed in the lateral decubitus position with the operated arm pulled by a traction device at ~4 kg. Generally, three portals were used for the procedure, that is, a posterior viewing portal and anteroinferior and anterosuperior portals. In cases that underwent additional SLAP repair, a trans-rotator cuff portal was used instead of the anterosuperior portal [9].
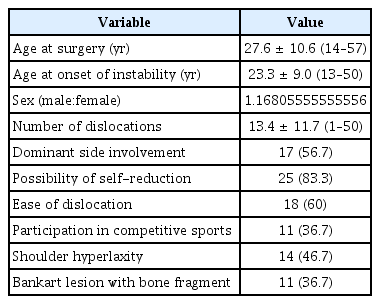
Diagnostic arthroscopy was performed prior to repair to determine the severity of glenoid bone loss and to confirm the presence of concomitant lesions. The anterior shoulder soft tissue stabilization procedure consisted of a Bankart procedure or anterior labroligamentous periosteal sleeve avulsion lesion fixation with capsular plication. For anteroinferior labral repair or anterior capsular shift, the abnormally-attached labrum or anteroinferior glenohumeral ligament was mobilized from the glenoid neck, and a 2–3 mm wide area of subchondral bone was exposed on the glenoid side for the recipient bed by removing articular cartilage using a motorized burr and curette. The capsule, together with the anterior labrum and bone fragment when present, were sutured and tacked down using three or four evenly spaced knotless suture anchors (Bioknotless; Depuy Mitek, Raynham, MA, USA or Pushlock; Arthrex, Naples, FL, USA) between 2 and 6 O’clock for the right shoulder. When present, bone fragments were always incorporated into the labral repair (Fig. 1). Incorporation of bone fragments was accomplished during capsulolabral reconstruction without extra procedures or devices and without increasing risk or technical difficulty.

Arthroscopic images in the lateral decubitus position viewed from the posterior viewing portal for the right shoulder. (A) Bankart lesion with a bone fragment (arrow) located medial to the articular surface. (B) The fragment (arrow) was reduced and incorporated into the capsulolabral repair. Comparison of preoperative and postoperative computed tomography (CT) images. (C) The bone fragment accompanying the Bankart lesion (asterisk) was visualized by preoperative CT. (D) Postoperative CT image showing healing of the fragment (asterisk) to the main glenoid rim.
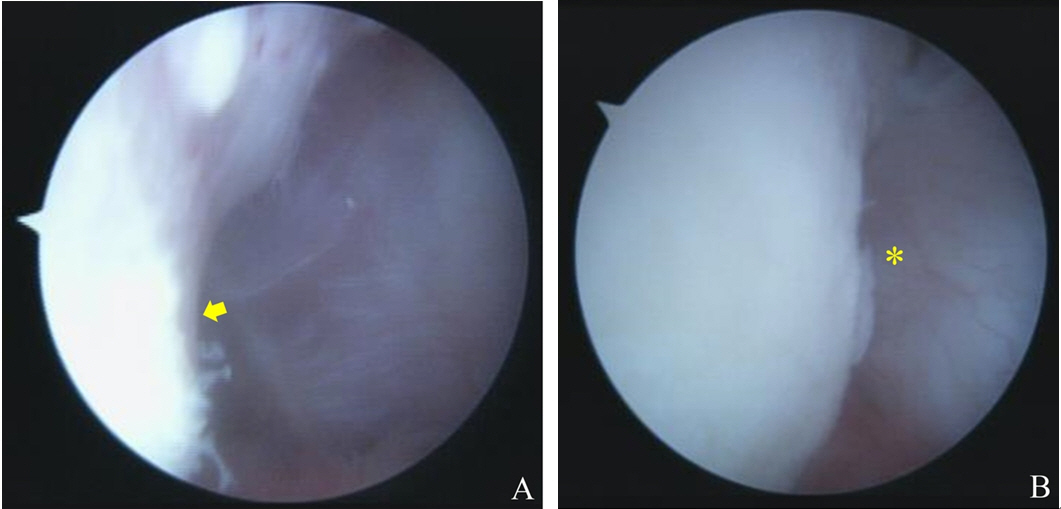
Procedures additional to anterior soft tissue reconstruction were incorporated as needed and included SLAP repair, remplissage, and/or rotator interval closure. Generally, SLAP lesions were repaired to add stability to glenohumeral joints [5], or to resolve painful instability as indicated by a positive SLAP physical test. To repair SLAP lesions, we used the trans-rotator cuff portal to achieve an appropriate angle for anchor insertion. After preparing the superior glenoid neck, and after anterior soft tissue reconstruction, one to two knotless anchors were used to repair the superior labrum (Fig. 2). To prevent postoperative external rotation limitation, the labral anterosuperior area (1 to 2 O’clock position) was not fixed.
(A) Arthroscope image showing a type II superior labral anterior and posterior lesion (asterisk) in conjunction with anterior labral detachment. (B) The lesion (asterisk) was repaired using two knotless suture anchors.
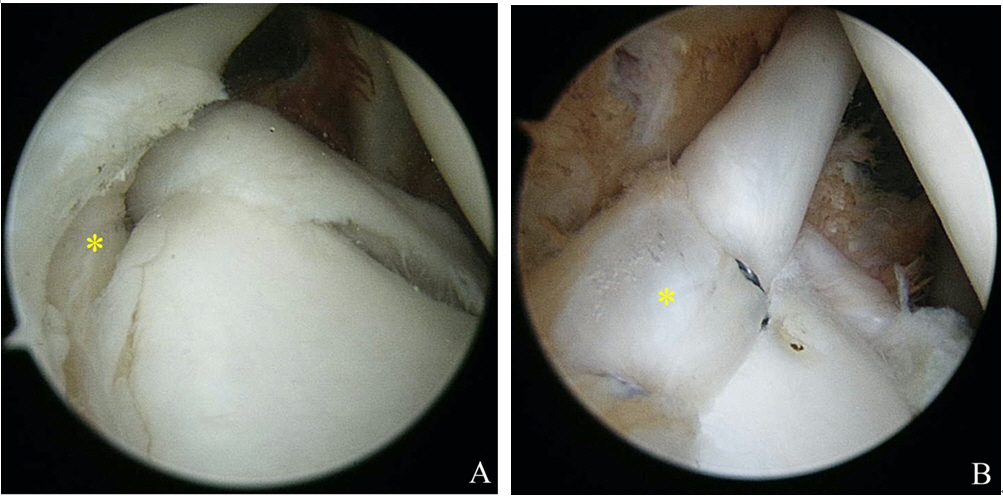
Remplissage was used for cases with significant Hill-Sachs lesions, which are defined as large nonengaging Hill-Sachs lesions that may engage in nonfunctional positions, but not engage during functional activities. This procedure allowed overriding of the humeral head on the anterior glenoid rim even after anterior soft tissue reconstruction (Fig. 3). For this procedure, the arthroscope was switched from the posterior to the anterosuperior portal, and a cannula was placed into the posterior viewing portal. The surface of the engaging Hill-Sachs lesion was gently freshened using a bur in reverse mode while taking care to remove a minimum amount of bone surface. One or two double-loaded suture anchors were then placed at the lesion margin using a penetrating grasper passed through the tendon and posterior capsule to grasp and pull one limb of the suture. These mattress sutures were then tied, such that knots remained within the extra-articular, sub-deltoid space, and filled in the infraspinatus tendon and capsule posterior to the Hill-Sachs lesion. Additional rotator interval closure was performed using additional suture anchors in cases with definite sulcus sign when the shoulder was placed in external rotation (Fig. 4).
(A) Arthroscope image showing a medially extended Hill-Sachs lesion (arrow) through the anterior portal. (B) The remplissage procedure was performed, and the posterior capsule was attached to the Hill-Sachs lesion (asterisk).
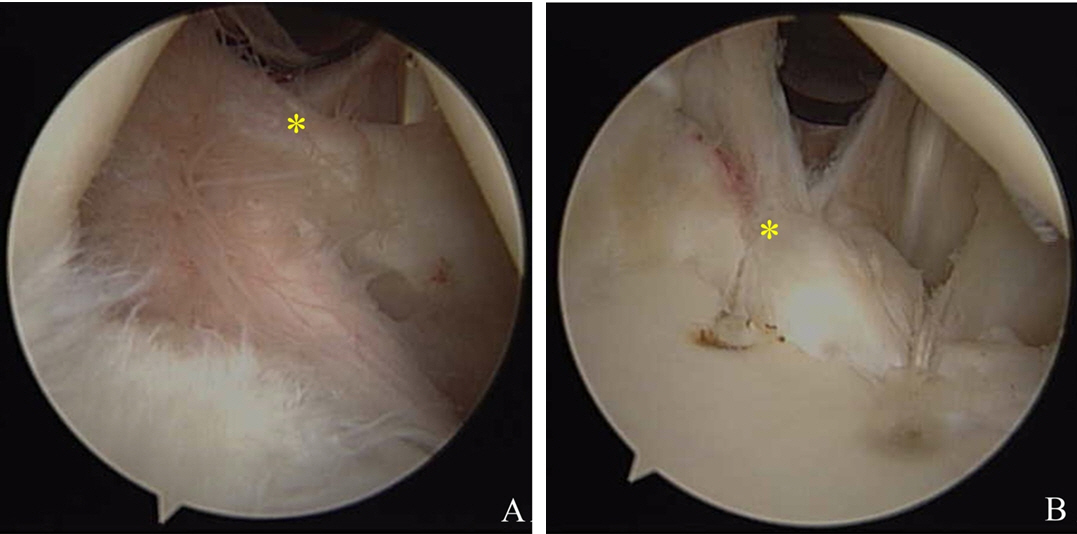
(A) Arthroscope image showing a wide rotator interval (asterisk) with the shoulder in external rotation. Preoperative joint laxity tests were positive for this patient. (B) Rotator interval closure (asterisk) was performed using suture anchors.
A standardized rehabilitation protocol was used throughout the study period. Strict immobilization in neutral shoulder rotation using an abduction pillow was maintained for 6 weeks. No motion was allowed during this period. Range of motion exercises were initiated at 6 weeks postoperatively when brace use was discontinued. Muscle-strengthening exercises, especially rotator cuff strengthening, were initiated at 3 months and continued until at least 6 months postoperatively. Return to sports was permitted at 6 months postoperatively.
Imaging Evaluation
The extent of glenoid bone loss (percentage and size in mm) were assessed using the best-fit circle method [10]. Initially, a best-fit circle was placed along the posterior and inferior margins of the glenoid in en face preoperative MRI or CT. A horizontal line was then placed through the center of the circle to extend from its posterior to anterior margins; this line represented the estimated diameter of the intact glenoid. A second horizontal line was then drawn at the same level between the anterior margin of the circle and the anterior margin of the glenoid; this line represented the amount of anterior glenoid bone loss (Fig. 5).

En face view of a three-dimensional reconstructed CT image used to measure the extent of a glenoid bone defect. A best-fit circle was drawn and localized on the inferior part of the glenoid using the PACS (Picture Archiving and Communication System). A horizontal line (the estimated diameter of the intact glenoid, green line) was placed within the center of the circle and extended from the posterior to the anterior margin of the circle. A second horizontal line (indicating the amount of anterior glenoid bone loss, red line) was placed at that same level between the anterior margin of the circle and the anterior margin of the glenoid.
Hill-Sachs lesions were classified as on-track or off-track using preoperative MRI [11], and defined as the region of cortical impaction along the posterosuperior margin of the humeral head. According to the on-track/off-track technique, the glenoid track is calculated using 0.84 D–d, where D represents the diameter of the intact glenoid (mm) and d corresponds to the amount of glenoid bone loss (mm) [6]. The Hill-Sachs interval is quantified as the sum of the width of the Hill-Sachs lesion (mm) and the width of the intact bone bridge (mm) between the rotator cuff attachment and the lateral margin of the Hill-Sachs lesion. Lesions were considered to be engaging or off-track if the Hill-Sachs interval exceeded the glenoid track, and to be non-engaging or on-track if the Hill-Sachs interval was less than the glenoid track (Fig. 6). All measurements were performed on axial images at the point of largest medial lesion extent. Measures and the determination of off-track or on-track status were performed independently by two fellowship-trained shoulder surgeons (SMR, WJ, JUK) with 1 and 2 years of experience, respectively.
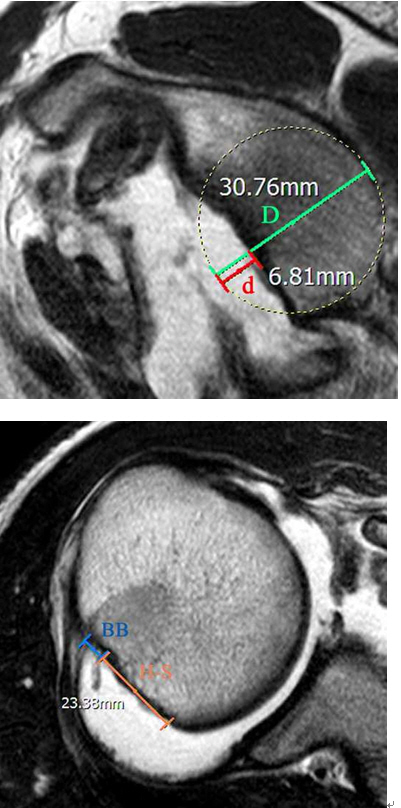
(A) Sagittal T2-weighted image showing a bone defect of the anterior glenoid margin. The green line indicates the estimated intact diameter of the glenoid (D, 30.76 mm), and the red line, the amount of glenoid bone loss (d, 6.81 mm). A best-fit circle was drawn in the lower two thirds of the glenoid. A glenoid track (0.84 D–d) of 19.03 mm was calculated. (B) Axial fat-suppressed T2-weighted image showing a broad superficial Hill-Sachs lesion. The Hill-Sachs interval measured 23.38 mm (the orange line indicates the Hill-Sachs lesion [HS], and the blue line the bony bridge [BB]).
Instability Severity Index Score Evaluation
Instability severity index score (ISIS) is a 10-point scale that evaluates six preoperative factors identified as being predictive of recurrent shoulder instability [7]. The four patient-related factors included are age <20 years (2 points), involvement in competitive sports (2 points), contact or forced overhead activities (1 point), and anterior or inferior hyperlaxity (1 point), and the two radiographic factors (identified on anteroposterior views) included are visible Hill-Sachs lesion on external rotation (2 points) and loss of normal inferior glenoid contour (2 points). An ISIS score of >6 points is considered the threshold for high risk of recurrence after only capsulolabral reconstruction [7]. Two fellowship-trained shoulder surgeons with 1 and 2 years of experience, respectively performed measures independently that were not affected by the results.
Statistical Analysis
Descriptive analysis was performed using the SPSS ver. 21.0 (IBM SPSS Corp., Armonk, NY, USA). The Wilcoxon rank sum test was used to evaluate differences between preoperative and postoperative values. P-values of <0.05 were deemed significant.
RESULTS
Conservative treatment was not attempted at either institution in patients with glenoid bone loss exceeding 20%. Demographic and preoperative data are presented in Table 1. The mean age at time of surgery was 27.6±10.6 years (range, 14–57 years) and mean age at onset of shoulder instability was 23.3±9.0 years (range, 13–50 years). Mean follow-up duration was 39.9±20.1 months (range, 24–86 months). Dominant sides were involved in 17 cases (56.7%), and instability symptoms in contralateral sides were noted in seven cases (23.3%). The average number of dislocation episodes that patients reported before surgery was 13.4±11.7 (range, 1–50), and hyperlaxity was positive in 14 shoulders (46.7%) [7,12].
Mean glenoid bone defect size was 25.8%±4.2% (range, 20.4%–37.2%) and >25% in 18 (60%) cases. Bone defects were measured on CT images in 22 cases (73.3%) and on MRI in the other eight cases (26.7%). For all 30 study subjects, mean ISIS score was 5.7±2.0 (range, 2–10) and 11 (36.7%) patients had a score of >6 points. Regarding on-track/off-track Hill-Sachs lesions, 21 patients (70%) had an off-track lesion. For all study subjects, the mean diameter of best fit circles around glenoid cavity perimeters was 26.7±1.7 mm, and the mean real anteroposterior dimension of the glenoid was 19.9±1.9 mm. Mean distance between rotator cuff insertions and medial borders of Hill-Sachs lesions was 17.7±5.3 mm. Bankart lesions with bone fragments were identified in 11 cases (36.7%), and in these cases, bone fragments and capsulolabral structures were repaired using suture anchors. SLAP lesions were identified in 12 cases (40%), and repaired in eight cases; debridement only was performed in two cases and tenotomy was performed in one case. Remplissage was performed in three cases only, and rotator interval closure in three cases. On average, 4.0±1.0 anchors (range, 3–6) were used for the repair, and more anchors were used in cases of concomitant SLAP repair. Three or more anchors were used for anterior capsulolabral reconstruction.
During follow-up, no obvious dislocations occurred in any patient, but a positive sensation of subluxation was reported by three patients. Interestingly, these three patients had off-track lesions as determined preoperatively, but only one had an ISIS score of >6. Revision surgery was not required in these three cases. Twenty-four (80%) of the study subjects returned to sports at their pre-injury levels. Among all study subjects, satisfaction with surgical treatment was 9.2±0.9 (range, 7–10) determined using a 10-point visual analog scale. Preoperative and postoperative ranges of motion and American Shoulder and Elbow Surgeons Shoulder and Western Ontario Shoulder Instability Index scores are listed in Table 2. Both increased significantly at the final follow-up. In addition, external rotation at 90° abduction and internal rotation at back was improved due to loss of a sense of instability by patients. Postoperative CT arthrography was performed in 21 patients (70%), and all showed evidence of satisfactory glenoid labrum healing.
DISCUSSION
This retrospective case series study demonstrates the feasibility of using an arthroscopic soft tissue procedure to treat anterior shoulder instability in cases with >20% glenoid defects. The definition of what constitutes a large or significant amount of bone loss is controversial, but based on recent consensus guidelines, a large defect is defined as 20%–30% loss of glenoid width and <21% loss of glenoid length [2-4]. The absence of recurrent instability among our cases is clinically relevant, as none of the 30 patients included in the sample required revision surgery and only three reported a sensation of subluxation during follow-up. Previous studies have reported recurrence rates after arthroscopic Bankart repair and capsular plication ranging from 4% to 21% and identified factors that contribute to differences in recurrence rates between studies and in variables including patient-specific characteristics, the definition of recurrence used (recurrent dislocation only or recurrent dislocation with subluxation), and follow-up duration [13,14]. Based on this information, the absence of any recurrence in our case series suggests that our soft tissue repair success rate was comparable to that of shoulder instability without bone defects, contrary to previously-reported outcomes for anterior shoulder instability with bone defects.
The presence of a glenoid bone defect in cases of anterior shoulder instability is one of the most important risk factors of recurrence [1,15]. Burkhart and De Beer [1] reported a 67% failure rate for arthroscopic Bankart repair in cases with significant bone defects, and a rate of only 4% in the absence of such defects. Boileau et al. [15] reported a 15% redislocation rate in the presence of >20% glenoid bone loss and found that this was also significantly associated with failures of soft tissue repair. Although several studies confirmed the influence of glenoid bone deficiency on the outcomes of anterior instability procedures [16,17], the critical cutoff value for bone deficiency has not been clearly determined. Recently, Ahmed et al. [18] reported that loss of >25% independently predicted failure, whereas Shin et al. [19] in a retrospective study found that a bone defect cutoff value of 17.3% best predicted recurrence after arthroscopic Bankart repair. However, Shin et al. [19] excluded Bankart lesions with bone fragments and did not provide any patient-specific information regarding sporting activity or occupation, which means that their results and ours cannot be directly compared. Kim et al. [20] reported a recurrent instability rate of 11% among patients with 20%–30% glenoid bone defects and moderate-to-low shoulder functional demands.
We agree that the Latarjet procedure and other bone graft procedures produce favorable outcomes in cases of anterior shoulder instability with significant bone loss. However, the Latarjet procedure is invasive, nonanatomic, technically demanding, and associated with considerable complications [21,22]. Although some clinicians have insisted that the Latarjet procedure is superior to Bankart repair for the treatment of anterior shoulder instability, regardless of bone defect [14], others have raised concerns regarding possible under-estimation and reporting of complications (e.g., screw problems, bone resorption, and later osteoarthritic changes) associated with the Latarjet procedure [23]. In fact, a recent systematic review reported a complication rate of 30% for the Latarjet procedure and recurrent dislocation and reoperation rates of 2.9% and 7%, respectively [23].
The recently-proposed glenoid track concept considers contributions of bone defects of the glenoid and head of the humerus to the biomechanics of anterior shoulder instability. Considering that 21 of our cases (70%) were off-track lesions, the rate of recurrent instability should theoretically have been higher, despite the fact we used only soft tissue repair rather than the bony procedure recommended for such cumbersome cases. However, on-track/off-track assessment considers only the rolling motion of the head of the humerus on the glenoid cavity as determined by static MRI [6], whereas this rolling motion is actually accompanied by a gliding motion during shoulder movement. As a result of this combined motion, during arm elevation in external rotation, the point of contact of the humeral head on the glenoid migrates from an inferior region to a superocentral-posterior region while the glenoid contact shifts posteriorly [24]. Given this posterior displacement of the head of the humerus, which would be increased by anterior capsulolabral reconstruction, the anterior glenoid rim and Hill-Sachs lesion may not contact each other, despite preoperative imaging findings of an off-track lesion. In addition, the importance of proprioception recovery after anterior stabilization needs to be considered, given that position sense and detection of movement should be equivalent to those of the uninvolved shoulder at 1 year after surgery [25]. From a clinical perspective, the ISIS scoring system was developed to predict failure of soft tissue repair, based on patient-related and radiographic characteristics, whereby a score >5 out of 10 is associated with a 70% risk of recurrent instability following arthroscopic anterior stabilization [6]. In a population-based study, an ISIS score of ≤6 points was associated with a 10% risk of recurrent instability after arthroscopic stabilization, and a score of >6 points was associated with a 70% risk. Those authors recommended open procedures, such as the Latarjet procedure, for cases with scores >6 points. In the present study, 11 cases (36.7%) had ISIS scores of >6 points.
The low rate of recurrence observed in the present study can be explained as follows. First, for the 11 cases (36.7%) with Bankart lesions and bone fragments, we incorporated fragment repair with the capsulolabral structure, and thus, when fragments healed, the sizes of glenoid bone defects were effectively reduced. Satisfactory results for bone fragment defect repair during arthroscopic capsulolabral reconstruction have been reported previously, even when those studies did not address larger bone defects [16,26]. In cases of attritional bone loss, defined as glenoid bone defects without bony fragments, outcomes of soft tissue repair are not as predictable [16], although the outcomes of such cases were clinically satisfactory in the present study. Second, we completely immobilized operated shoulders for 6 weeks, and even forbade pendulum motion, passive exercise, and return to sports activity during this period. Furthermore, before returning to sports activities, patients were instructed to perform extensive rotator cuff strengthening exercises that commenced 3 months after surgery and continued until at least 6 months postoperatively. Extensive rotator cuff strengthening exercise started with isometric exercises with resisted contraction for internal and external rotation, and then added isotonic shoulder strengthening with rubber bands. Somewhat surprisingly, no guideline studies have evaluated the effects of different postoperative rehabilitation protocols on the results of shoulder instability surgery. Nevertheless, the conservative rehabilitation protocol that we adopted could have affected our results. Third, three or more anchors were used in all cases to stabilize anterior capsulolabral structure, as the use of less than three anchors has been shown to be a risk factor of recurrence [15].
The reliability and validity of glenoid bone defect identification and Hill-Sachs lesion measurements must also be considered. Direct visual measurement during arthroscopic surgery provides the most reliable data in most cases, but in 48% of cases, bare bone is not well visualized. Moreover, referencing a bone defect to the center of the glenoid is possible in only about 37% of cases, as the referencing landmark is eccentrically located in the other 63% [27]. To overcome these issues, the use of three-dimensional (3D) CT is recommended as the gold standard to measure glenoid bone loss, as 3D CT measurements have been reported to be well correlated with arthroscopic measurements and to have high reliability based on cadaveric measurements [28]. In our case series, glenoid bone loss was measured using MRI in several cases, due to the absence of 3D CT images. Although 3D CT is considered the gold standard, glenoid bone loss can be accurately measured on MRI using the circle method, which has been shown to compare favorably with 3D CT and CT measurements [29]. Recently introduced on-track/off-track measurements hold promise as feasible clinical measures of bone loss, but are currently limited with respect to the measurement of Hill-Sachs deficits and by the ambiguous measurement guidelines provided in the original publication describing their use [30].
The importance of the effects of glenoid bone defects on anterior shoulder stability is being increasingly recognized, and this recognition has expanded the use of the Latarjet procedure. Although the Latarjet procedure does provide definite shoulder stability in patients with glenoid bone defects, we contend that anterior capsulolabral reconstruction is probably sufficient in the majority of cases. Sometimes, over-treatment is more problematic than under-treatment since the former is more invasive and associated with higher complication rates. Therefore, we suggest that selection of the best surgical strategy should be an individualized decision based on careful discussion with the patient, rather than being based solely on objective considerations of bone defect size.
This study has several limitations that require consideration. First, it is inherently limited by its retrospective case series design, the small number of cases included, and by the lack of a control group. A retrospective, case controlled study would have been more relevant, but at the two involved institutions, soft tissue procedures are used as first-line treatments for anterior instability even in patients with >20% glenoid bone loss. Furthermore, it should be noted that >20% loss of glenoid contour in cases of anterior shoulder instability is relatively uncommon; the rate of occurrence was only 9.9% in our case series. Second, our incorporation of additional procedures was not standardized. Most frequently, anterior instability was accompanied by a SLAP lesion (43.8% of cases). However, SLAP repair was performed in only nine of these cases, while debridement was performed in four cases and tenotomy in the remaining case. Remplissage and rotator interval closure were performed in three cases each. For SLAP repairs, the superior labrum was repaired to relieve symptoms and to add shoulder stability [31]. Therefore, debridement and tenotomy were performed for cases in which extents of injury to labrum and biceps tendon were deemed insufficient to warrant full SLAP repair. Remplissage and rotator interval closure were performed at the surgeon’s discretion. We were not able to evaluate whether adding these procedures enhanced shoulder stability. As previously mentioned, bone defects were measured using preoperative MRI in nine cases rather than using 3D CT images, which is the recommended gold standard [29].
This retrospective case series analysis of 30 cases of anterior shoulder instability with glenoid bone defects exceeding 20% indicates that the use of arthroscopic soft tissue repair is feasible and provides clinically favorable outcomes, even in a sporting population. This finding is important when one considers the invasive nature of the Latarjet procedure and its high rate of associated complications. Future studies should focus on determining the size range of bone defects that can be successfully managed by soft tissue repair.
Notes
Financial support
None.
Conflict of interest
None.