Ultrasound-guided needle decompression and steroid injection for calcific tendinitis of the shoulder: risk factors for repeat procedures and outcome analysis
Article information
Abstract
Background
Although ultrasound-guided needle decompression (US-GND) can treat calcific tendinitis of the shoulder effectively, repeat procedures might be required for unresolved symptoms. We evaluated the overall clinical outcomes of US-GND with subacromial steroid injection and the final results and factors predisposing toward repeat procedures.
Methods
Ninety-eight patients who underwent US-GND for calcific tendinitis of the supraspinatus/infraspinatus were analyzed between March 2017 and December 2018. The clinical outcomes (pain visual analog scale, functional visual analog scale [FVAS], and American Shoulder and Elbow Surgeons [ASES] score) and final subjective satisfaction were compared between groups A (single US-GND) and B (repeat US-GND). The factors predisposing toward repeated US-GNDs were analyzed.
Results
We found that 59.3% (58/98) of patient ASES scores were ≥80, and 73.5% of patients (72/98) were satisfied with the outcome. Group B (n=14) demonstrated a significantly higher rate of dominant-arm involvement compared to group A (78.6% vs. 48.8%, p=0.046). However, initial calcification size, shape, number, density, subscapularis involvement, lavage, and procedure time did not differ significantly between the groups. Group B showed poorer final FVAS (7 [interquartile range, 6–8] vs. 8 [interquartile range, 7–9], p=0.036) and subjective satisfaction compared to group A (satisfied: 5 [35.7%] vs. 67 [79.8%], p<0.001].
Conclusions
US-GND with subacromial steroid injection is a viable treatment option for calcific tendinitis of the shoulder. Dominant-arm involvement was the only independent factor for repeated US-GND. Final outcome of repeated US-GND for unimproved patients was promising; however, these outcomes were poor compared to those of the patients who improved after the first procedure.
INTRODUCTION
Calcific tendinitis is the most common pathology identified in patients with shoulder pain, with a prevalence that ranges from 6.8% to 54% [1-4]. Although calcific tendinitis is a self-limiting disease, it sometimes can require intervention because of severe pain [3,5]. The initial treatment options for calcific tendinitis include pain control with oral medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), and injection of corticosteroids into the shoulder joint [3]. However, calcific deposits can be removed using real-time ultrasound-guided needle decompression (US-GND) if patients have consistent pain with prominent calcific deposits on plain radiography [6].
US-GND possesses several advantages. US-GND can be used to directly remove the calcific deposits without surgery. The procedure is convenient and not burdensome for either clinician or patient since US-GND can be performed under local anesthesia, enabling faster recovery [7]. Numerous studies have reported good clinical outcomes after US-GND [3,8,9]. The success rate for US-GND is approximately 70% [8,10-12]. Moreover, US-GND is the most effective treatment for calcific tendinitis of the shoulder among the various treatments (extracorporeal shock wave therapy [ESWT], US-GND, corticosteroid injection, and combined treatment) according to a systematic review by Arirachakaran et al. [13]. However, some studies reported that up to 42% of patients had persistent shoulder complaints after undergoing US-GND [4,14,15]. Therefore, the outcome of US-GND for calcific tendinitis is being debated.
Previously, repeat procedure is among the commonly reported factors predisposing toward poor clinical outcomes after US-GND [4,14-16]. Farin et al. [10] reported that repeated US-GND resulted in poor final outcomes compared to single US-GND. However, the predisposing factors associated with reluctance to undergo single US-GND requiring repeat procedure and the detailed outcomes of repeated US-GND such as pain, function, results of additional magnetic resonance imaging (MRI), and the need for surgical removal have not been explored.
Hence, this study aimed to analyze clinical and radiologic outcomes of US-GND at our institution, predisposing factors for repeated US-GND, and final outcome of repeated US-GND compared to that of single US-GND. The hypotheses were that US-GND would show good clinical and radiologic outcomes (comparable to previous studies), repeat US-GND would induce poor clinical and radiologic outcomes compared to single US-GND, and there are predisposing factors associated with repeated US-GND.
METHODS
This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2020-02-121-001). The need for informed consent was waived owing to the retrospective study design, which posed minimal risk to the patients during data acquisition.
Patients with painful calcific tendinitis refractory to conservative treatment, including medication, steroid injection, and ESWT, exceeding 6 months who underwent US-GND between March 2017 and December 2018, were assessed retrospectively. During this period, a total of 125 patients underwent US-GND for calcific tendinitis of the shoulder at our institution. Patients who had calcific deposits larger than 3 mm on the supraspinatus (SSP) or infraspinatus (ISP) tendon on any radiographic view and who underwent US-GND were included in this study.
Patients with the following conditions were excluded: (1) calcific deposits only in the subscapularis (SSC) tendon (n=6); (2) dystrophic calcification according to the French Society of Arthroscopy (FAS) classification [17] (n=4); (3) combined full-thickness rotator cuff tear on US imaging (n=2); (4) history of previous US-GND (n=1), surgery (n=1), fracture (n=2), or inflammatory arthritis including osteoarthritis (n=3), rheumatoid arthritis (n=1), and infectious arthritis (n=0) of the affected shoulder; and (5) less than 6 months of follow-up (n=7). After exclusions, 98 patients met the criteria and were involved in this study.
Clinical and Radiologic Evaluation
The shoulder range of motion (ROM), visual analog scale for pain (PVAS), VAS for function (FVAS), and American Shoulder and Elbow Surgeons (ASES) scores [18] were evaluated to assess the clinical outcome. The shoulder ROM included active forward elevation (FE), external rotation (ER) to the side, and behind-the-back internal rotation (IR). The FE and ER were measured using a goniometer. A 10-point scale was used to measure the IR based on the following anatomic levels described by Levy et al. [19]: greater trochanter to the buttocks (2 points), sacrum to L4 (4 points), L3 to L1 (6 points), T12 to T8 (8 points), and T7 to T1 (10 points). All functional scores were recorded by a single athletics shoulder trainer (SML) blinded to the study, and the ROM was assessed by five shoulder fellows trained at our institution.
Radiologic evaluation was performed using plain radiography (anteroposterior, true anteroposterior, cephalic tilt, axillary lateral, and arch view), which was obtained with the patient shoulders in neutral rotation. The maximum length of the deposit was measured on any plain radiograph. The number of calcific deposits was designated as single or multiple. Clustered lobular calcifications consisting of one clump were designated as a single deposit (Fig. 1). The morphology of the calcific deposits was assessed using the FAS classification: type A (sharply delineated, dense, and homogenous deposits); type B (sharply delineated, dense appearance, with multiple fragments); and type C (heterogeneous appearance, fluffy deposit). Patients with type D deposits were excluded (Fig. 2) [17]. The qualitative reduction in the calcific deposits was assessed after the procedure based on comparison with baseline: 1, no change; 2, less than half removed (<50%); 3, approximately half removed (about 50%); 4, more than half removed (>50%); and 5, completely removed (Fig. 3). All radiologic measurements were performed by two trained shoulder fellows (SCK and KSC) who were blinded to the result of the study, and the inter-observer reproducibility was calculated.
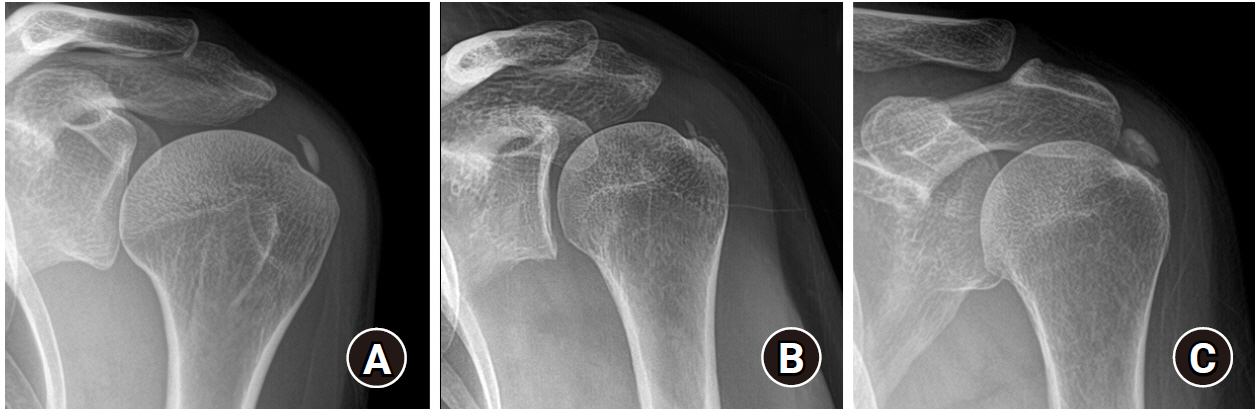
Number of calcific deposits: (A) single, (B) multiple; (C) clustered lobular-shaped deposits were regarded as a single entity.
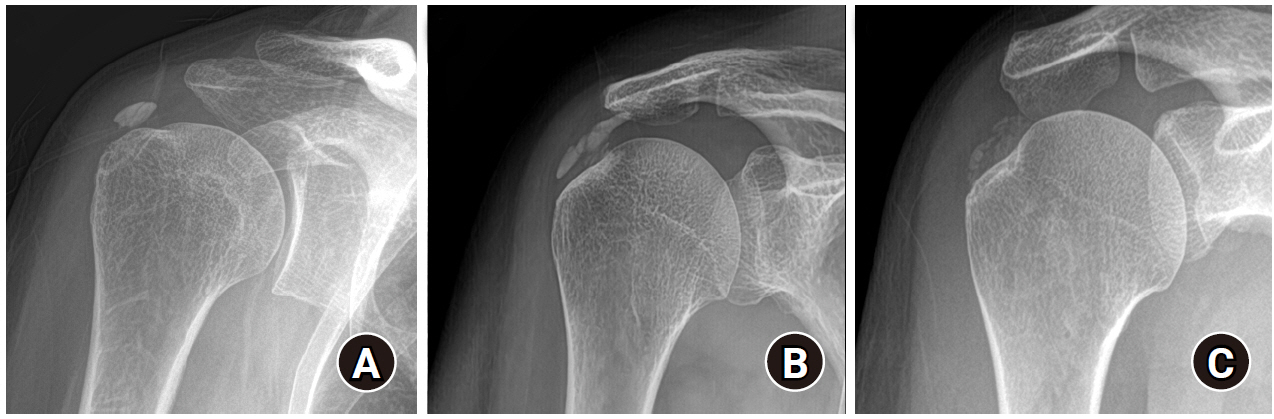
French Society of Arthroscopy classification (A) type A (sharply delineated, dense, and homogenous appearance of deposits), (B) type B (sharply delineated, dense appearance, and multiple fragments), (C) type C (heterogeneous appearance, fluffy deposit).
Qualitative reduction in a calcific deposit (A) less than half removed, (B) approximately half removed, (C) more than half removed, (D) completely removed.
Clinical and radiologic evaluations were performed before and after the procedure at each outpatient visit. The patients were followed up for 2 months after the US-GND; the number of subsequent outpatient visits varied depending on improvement in symptoms. US-GND was repeated up to three times if the patient had persistent symptoms and calcific deposits on plain radiography. At the last outpatient visit, the patients were asked final questions about subjective satisfaction, additional MRI, and whether operated or not. Subjective satisfaction was divided into four categories: very satisfied, satisfied, the same, and poor. If the patients did not visit the outpatient clinic for more than 6 months, a telephone survey was conducted to ascertain the PVAS, FVAS, ASES scores, and the answers to final questions.
Ultrasound-Guided Needle Decompression
All procedures were performed at our institution by five first-year shoulder fellows during the study period. The patients were required to sit on a bed with a 70° backrest, and the affected shoulder was exposed for diagnostic US for calcific tendinitis and rotator cuff pathology. The arm was positioned in IR if the calcific deposit was located in the SSP/ISP tendons. For SSC calcifications, the arm was positioned in ER.
The skin over the lesion was sterilized using betadine and alcohol, and a sterile O-hole drape was placed. Sterile US gel and film were used. Local anesthesia (2% lidocaine hydrochloride [400 mg/4 mL, Daihan Pharm, Seoul, Korea]) was injected into the skin and around the calcific deposit under real-time U.S. guidance to reduce pain during the procedure. The calcific deposits were punctured repeatedly with an 18-G needle until all hard sections of the calcific deposit were softened and lost any definitive shape. During the US-GND, density of calcification, possibility of lavage, and operative time were recorded. When the calcific deposit was firm and difficult to fragment, we recorded the finding as “hard.” When there was no resistance when passing the needling through the calcific deposit, we recorded the finding as “soft.” The density between these was recorded as “intermediate.” After puncture, the calcific debris were aspirated using a 10-mL syringe with a new 18-G needle, if possible.
After the procedure, a mixture of 4 mL of 1% lidocaine and 1 mL of triamcinolone acetonide (40 mg/1 mL; Hanall Pharm, Seoul, Korea) was injected into the subacromial space, and oral NSAIDs were prescribed for 1 month. Patients were instructed to move their shoulder immediately after the procedure without any restriction in motion.
Statistical Analysis
All initial and final clinical and radiologic outcomes were analyzed using paired comparisons. Patients were divided into two groups depending on whether they received one (group A) or more than one (group B) procedure, and the variables of Groups A and B were compared. Moreover, patients were divided into “satisfied vs. dissatisfied” and “no calcific deposit vs. remnant calcific deposit” groups at the final examination, and their variables were compared.
All statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC, USA). Continuous variables (age, symptom duration, follow-up duration, calcific deposit size, operative time, ROM, and functional scores) were analyzed using Student t-test or Mann-Whitney-Wilcoxon test. Categorical variables (sex, site of involvement, dominant-arm involvement, diabetes, smoking status, SSC involvement, number of deposits, FAS classification, lavage, density, decrease in calcific deposit, and final questionnaires) were analyzed using the chi-square test or Fisher’s exact test. Multivariable logistic regression analysis for repeated US-GND was performed. An alpha value of 0.05 was used.
Interobserver reproducibility for the radiologic measurements was calculated using Cohen's kappa coefficient (κ) for categorical variables (FAS classification, qualitative reduction in the calcifications) and the intraclass correlation coefficient (ICC) for continuous variables (size). If the ICC or κ was >0.75, 0.4–0.75, or <0.4, the reliability was considered excellent, fair to good, or poor, respectively. The interobserver reproducibility for the radiologic variables was: the ICCs for initial and final calcification size were good (0.90, 0.95), κ for final reduction in calcification size was good (0.80), and κ for the initial and final FAS classification was fair to good (0.51 and 0.62).
Post hoc power analysis was performed to determine the statistical power for comparison between groups A and B. The ASES score at the final follow-up was the primary outcome of this study. A minimal clinically important difference of 12 [20] and standard deviation of 15 [6,8] in ASES scores were determined as per prior studies, and the significance level was set to an alpha value of 0.05. The comparison between group A (n=84) and group B (n=14) yielded a statistical power of 79.2%.
RESULTS
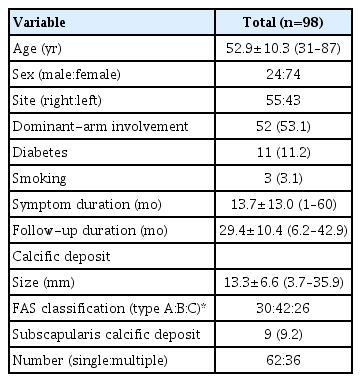
Ninety-eight patients (mean age, 52.9±10.3 years) with 29.4±10.4 months follow-up were analyzed. Initial calcific deposit size was 13.3±6.6 mm, and FAS type B (42.9%) was the most commonly observed. The demographic and initial characteristics of the calcific deposits of the consecutive patients are presented in Table 1.
During the first US-GND, 36 dense, 39 intermediate, and 23 soft calcific deposits were observed. Saline lavage was possible in 55 patients, and the mean procedure time was 29.1±11.3 minutes (range, 13–47 minutes). After the first US-GND, 14 patients (14.3%) underwent repeated US-GND (group B), and 1 patient underwent US-GND three times. The mean period between initial and second US-GND was 4.2±2.2 months (range, 1.4–9.7 months), and the procedure time for the repeat procedure was 16.5 minutes (interquartile range, 12.8–28.0 minutes).
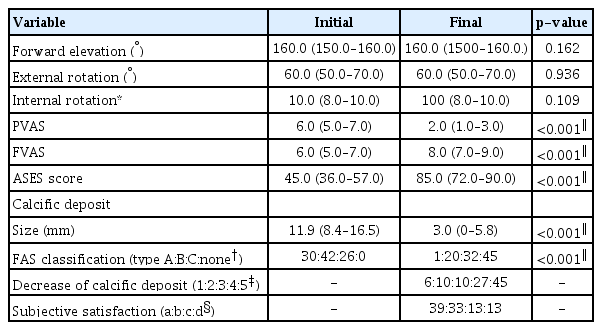
The overall clinical and radiologic outcomes are presented in Table 2. All functional scores improved significantly in the final follow-up compared to the initial. The calcific deposits were removed completely in 45 patients (45.9%), and 72 patients (73.5%) were very satisfied or satisfied. Nine patients (9.2%) underwent additional MRI, and all patients showed more severe than grade two partial tears of the SSP tendon [21]. Among these nine patients, four (4.1%) underwent arthroscopic calcific material removal and rotator cuff repair.
Comparison between Needling Once and Repeated Needling
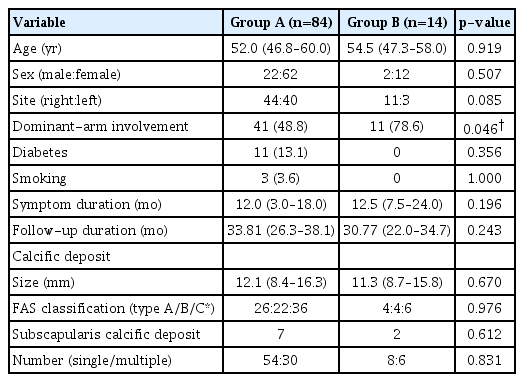
The patients’ demographics and initial characteristics, including the ROM and functional scores, did not differ significantly between groups A and B with the exception of dominant-arm involvement (Table 3).
The initial size of the calcific deposit, shape according to the FAS classification, number of deposits, SSC involvement, density (group A: 30 dense, 33 intermediate, 21 soft; group B: 6 dense, 6 intermediate, 2 soft; p=0.761), saline lavage (group A: 48, group B; 7, p=0.835), and procedure time (group A: 29.0 minutes [interquartile range, 19.8–35.3]; group B: 29.5 minutes [interquartile range, 18.0–45.8], p=0.583) did not differ significantly between groups A and B.
In multivariable regression analysis of repeated needling, dominant arm involvement was an independent predisposition factor (odds ratio, 4.075;, 95% confidence interval, 1.033-16.078, p=0.045), but symptom duration was not (odds ratio, 4.075; 95% confidence interval, 1.033–16.078, p=0.045), but symptom duration was not (odds ratio, 1.031; 95% confidence interval, 0.991–1.074, p=0.130).
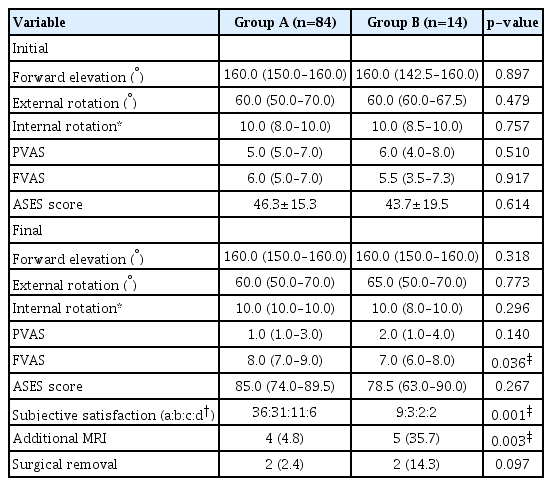
The clinical outcomes of groups A and B are presented in Table 4. Final FVAS scores and subjective satisfaction were significantly better in group A (50% satisfied) than in group B (35.7% satisfied). The frequency of additional MRI was significantly higher in group B. The reason for the MRI scan of four patients in group A was that SSP partial tear was suspected on the ultrasound during US-GND. However, persistent pain in the affected shoulder in five group B patients resulted in performance of MRI scans. However, the rate of surgical removal did not differ significantly between the two groups.
Change in Calcific Deposits
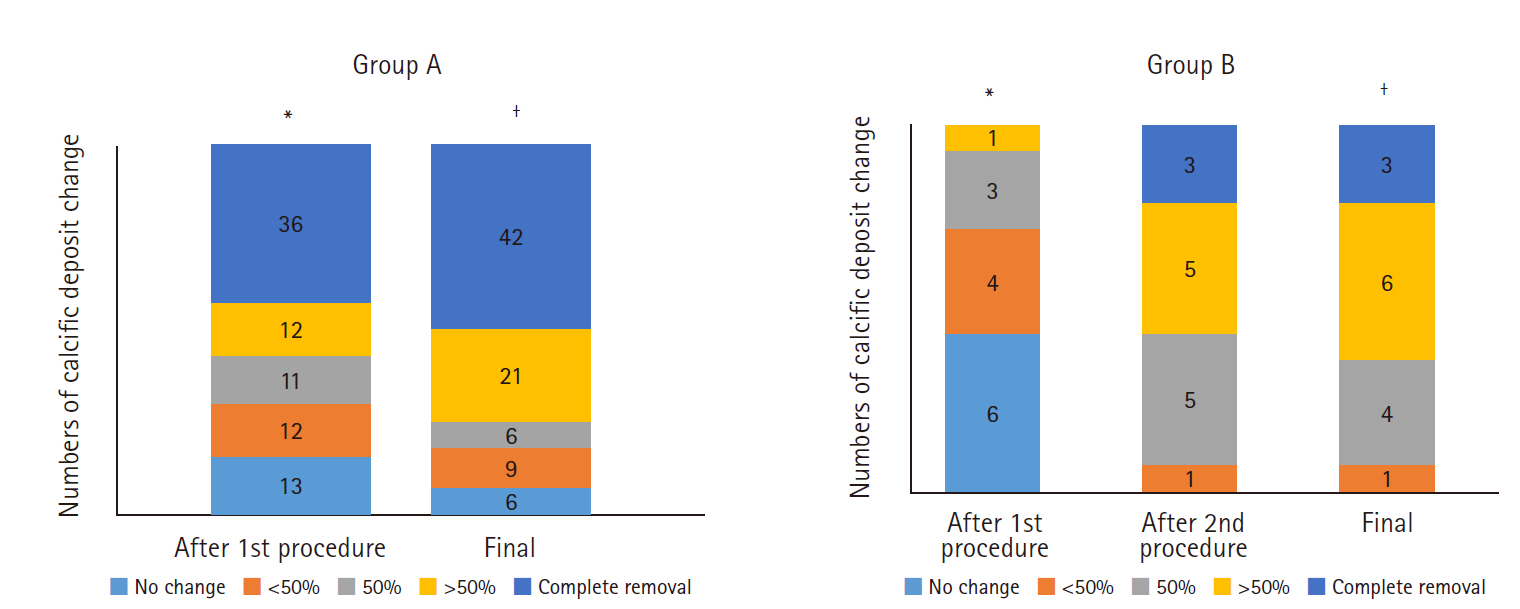
The change in size of the calcific deposits according to number of needling procedures in groups A and B is described in Fig. 4. The size of the calcification after the first needling procedure was 4.1±4.7 mm and 9.5±4.7 mm in groups A and B (p<0.001), respectively. After the second needling procedure, the size of the calcific deposits decreased to 5.5±4.7 mm in group B, which did not differ from the post-procedural size of group A (p=0.207). The final deposit size did not differ between the groups (p=0.274).
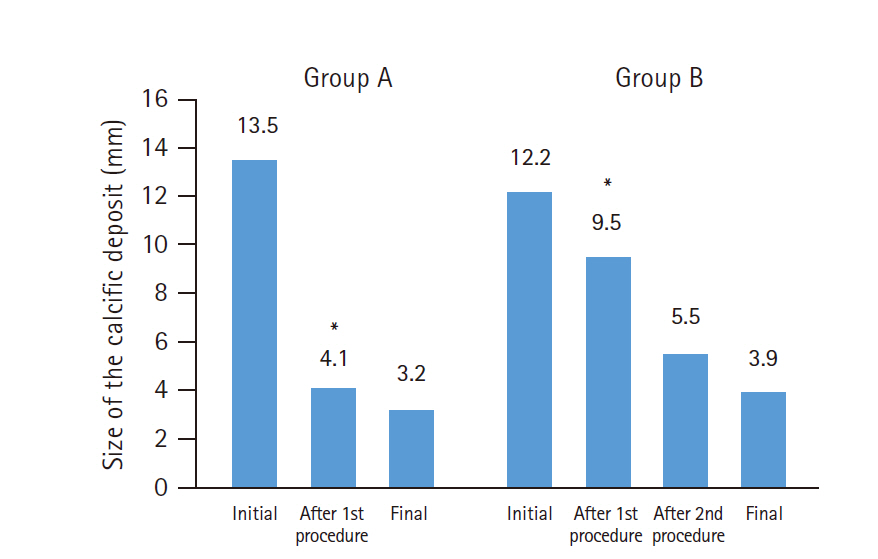
Mean change in the size of the calcific deposit. Postop: postoperative. *Significantly different (p<0.001).
The changes in the FAS classification in groups A and B are shown in Fig. 5. Initially, type B was most common in groups A and B. After the needling procedure in group A, 36 patients had no calcification; and type C calcifications were observed most commonly. Complete removal of the calcific deposits after the first procedure was not observed in any patient in group B. Finally, type B was most commonly observed (n=6) and complete removal of calcification was observed in three patients in group B. The final FAS classification differed significantly between the two groups (p=0.039).
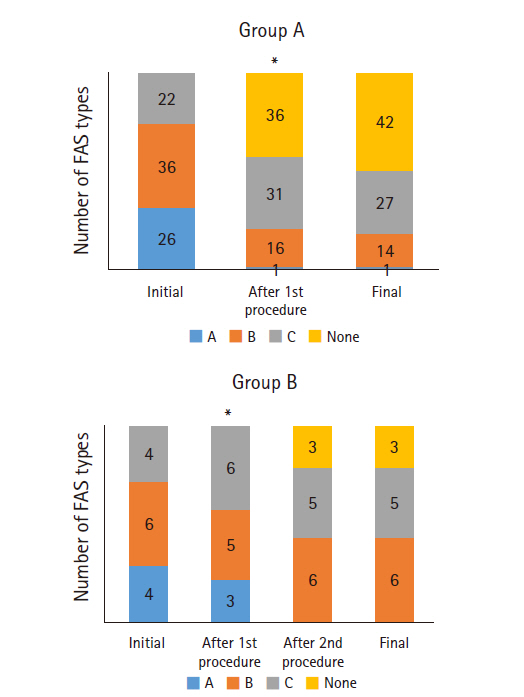
Change in French Society of Arthroscopic classification. Postop: postoperative.*Significantly different (p<0.001).
The reduction in calcific deposits is presented in Fig. 6. After the first procedure, 36 patients in group A showed no calcification and 12 patients showed greater than 50% calcification reduction. After final removal, 42 patients in group A showed no calcification and 21 patients showed greater than 50% calcification reduction. In group B, one 1 patient showed a reduction in size exceeding 50% after the first procedure, and eight showed a reduction exceeding 50% after the second procedure.
Comparison According to Final Subjective Satisfaction
At the final follow-up, 72 patients were very satisfied or satisfied, while 26 patients were not satisfied (Table 5). The initial calcification size was significantly larger and the final functional scores were better in the satisfied patients compared to the dissatisfied patients.
Comparison According to Residual Calcific Deposit
The calcific deposits were completely removed in 45 patients (group CR), while 53 patients had residual deposits (group RD). The initial characteristics did not differ between groups CR and RD, and only initial shape according to the FAS classification differed significantly between the groups (group CR vs. RD: type A:B:C, 17:13:15 vs. 13:29:11; p=0.036). However, final ROM and functional scores were not different between the groups.
DISCUSSION
This study analyzed the overall clinical and radiologic outcomes of US-GND for calcific tendinitis of the shoulder over a minimum follow-up period of six months. Repeated US-GND was performed in 14.3% of patients; overall, 73.5% of patients were satisfied at final follow-up. Patients who underwent repeated US-GND showed a significantly higher rate of dominant-arm involvement, poor final FVAS and subjective satisfaction, and a higher frequency of additional MRI evaluation for rotator cuff pathology compared to those who underwent US-GND once. Based on final subjective satisfaction, dissatisfied patients had smaller calcifications compared to satisfied patients. However, the presence of residual calcific deposits on final plain radiograph was not associated with clinical outcome.
Although the specific methodology differed by clinician, the previously reported clinical success rate after US-GND was approximately 70% [8,10-12], and US-GND was a good treatment modality for calcific tendinitis of the shoulder compared to other options [3,13,22]. Kim et al. [3] reported that US-GND elicited superior clinical and radiologic outcomes for treatment of calcific tendinitis of the shoulder compared to ESWT. Oudelaar et al. [22] reported that 74% of patients experienced complete symptomatic relief at 6 months. Moreover, a recent meta-analysis reported that the needling procedure was the most efficient treatment method according to the clinical outcomes obtained after 2 years’ follow-up [13].
However, some studies have shown that US-GND does not guarantee good outcomes for calcific tendinitis of the shoulder [14,15,22,23]. The improvement in pain at one year after US-GND reportedly ranged from 51% to 69% according to a systematic review [24], and more than 42% of patients complained of recurrent shoulder pain after the procedure [14,22]. Moreover, 18%–45% of patients underwent repeat US-GND owing to persistent pain in the affected shoulder after the first procedure [10,23]. de Witte et al. [15] compared the clinical and radiologic outcomes between US-GND and subacromial steroid injection alone and reported no difference between the two treatment modalities.
In our study, the mean size of the calcific deposit decreased significantly after US-GND, and 67.3% of patients had PVAS score ≤2, 59.3% had ASES score≥80, and 73.5% were satisfied to very satisfied. Thus, the success rate of US-GND in our study is comparable to that of previous studies, which was approximately 70% [6,8,10,11]. Repeated US-GND was performed in 14.3% patients in our study, which was slightly lower than that reported in previous studies (18%–45%) [10,23].
Previously, repeat procedures have been reported as a common poor prognostic factor for US-GND. [4,25]. Ogon et al. [25] reported that small deposit size and repeated needling procedures were associated with poor outcomes; and Oudelaar et al. [4] reported that long symptom duration, repetitive procedures, and smaller calcific deposits were poor prognostic factors for US-GND.
In our study, only dominant-arm involvement was an independent risk factor for repeat needling, consistent with de Witte et al. [14]. Other factors such as initial calcific deposit size, shape, number, density, possibility of lavage during the procedure, and procedure time were not associated. The reason for this observation was not revealed by our study. However, we assumed that the relatively greater use of the dominant arm might be the cause of persistent symptoms and consequent requirement for repeated needling procedures.
Patients who underwent repeated US-GND showed low ROM and functional scores similar to those of previous studies, even though only FVAS was statistically significant [4,10]. However, as mentioned by Oudelaar et al. [4], the reason for the poor level of satisfaction could not be clearly identified; it was unclear whether the repeat needling procedure rendered the patients unhappy or if dissatisfied patients underwent repeated needling procedures.
Although the initial calcification size was not associated with repeat US-GND, there was a significant difference of initial calcification size between the satisfied and dissatisfied patients. Dissatisfied patients showed smaller initial calcifications compared to their satisfied counterparts. However, the relationship between initial calcification size and result of the needling procedure is inconclusive [4,25]. Oudelaar et al. [4] asserted that large calcifications exert a predominantly space-occupying effect; however, small calcifications seem to have more inflammatory symptoms than space-occupying effects. Moreover, patients with small calcifications had fewer complaints than those with large calcifications; thus, the effect of the needling procedure was smaller than that for large deposits.
Furthermore, complete removal of calcific deposits is associated with US-GND outcomes [15,26]. Krasny et al. [26] reported that patients with gradual radiologic improvement showed better clinical outcomes. Further analysis was conducted to determine if the final residual calcification was related to the final outcome; however, no association was observed in this study. Therefore, complete removal of the calcific deposit seems unnecessary, and the decision to repeat US-GND should not be based solely on radiographs.
In the early period at our institution, radiologists performed the US-GND by consultation. However, since 2012, first-year orthopedic shoulder fellows have been performing the US-GND. We had concerns over the outcomes when the practitioner changed; however, this study revealed no great difficulty in learning the US-GND procedure for orthopedic doctors. These doctors achieved good outcomes.
Moreover, the mean procedure time was 29.1 minutes, which is fairly long for US-GND. Although none of the previous studies have reported on the association between procedure time and US-GND outcomes and no association was observed in our study, we opine that puncturing the calcific deposit for a sufficient duration is vital to achieving good results.
This study has several limitations. First, there were limitations inherent to the retrospective study design. The number of patients requiring repeat needling was insufficient because the frequency of the repeated needling procedure is relatively low. Although the inclusion of 14 patients with repeated US-GND yielded a statistical power of 79.2%, a larger sample is required to obtain clearer outcomes for repeated US-GND. Second, the follow-up duration was short, with a minimum period of six months. Some difficulties were encountered in motivating the patients to revisit the outpatient clinic for long-term follow-ups since some patients experienced marked improvements in pain and function after the procedure. Patients whose symptoms improved after the first needling could not be assessed regularly at the outpatient clinic. However, the mean follow-up period was 29.4 months, which is sufficient to assess the results of US-GND. Third, the final follow-up duration ranged widely from 6 to 42 months after the needling procedure. Calcific deposits usually dissolve and disappear over time after needling, and the patients’ symptoms also change over time [22]. Hence, comparing the radiologic and clinical outcomes of the needling procedure with different follow-up durations could have been fallacious. Fourth, other treatment after US-GND was not analyzed. In particular, NSAIDs were prescribed for 1 month after the procedure, but drug compliance was not recorded due to the retrospective research design. Drug compliance and additional treatment might have affected clinical results; therefore, these are potential confounding factors. Finally, the criteria for calcific density evaluation might have been subjective for practitioners.
US-GND with subacromial steroid injection is a good treatment option for calcific tendinitis of the shoulder. Dominant-arm involvement was the only factor associated with repeated US-GND, while the size, shape, number, and density of the calcifications and possibility of lavage were not associated factors. The final outcome of repeated US-GND for unimproved patients was also promising; however, the function and subjective satisfaction were poor compared to those of the patients who improved after the first procedure.
Notes
Financial support
None.
Conflict of interest
None.