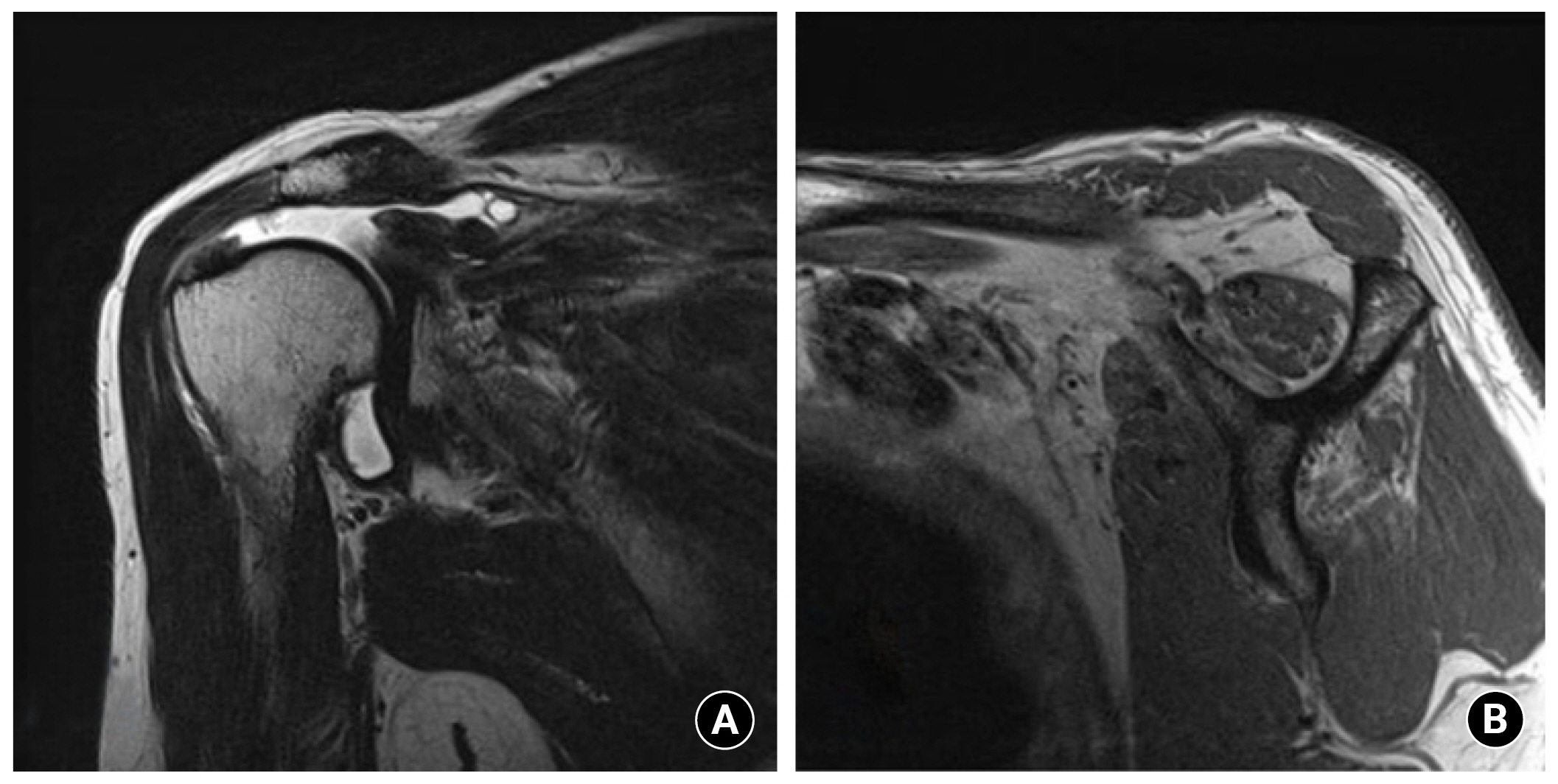
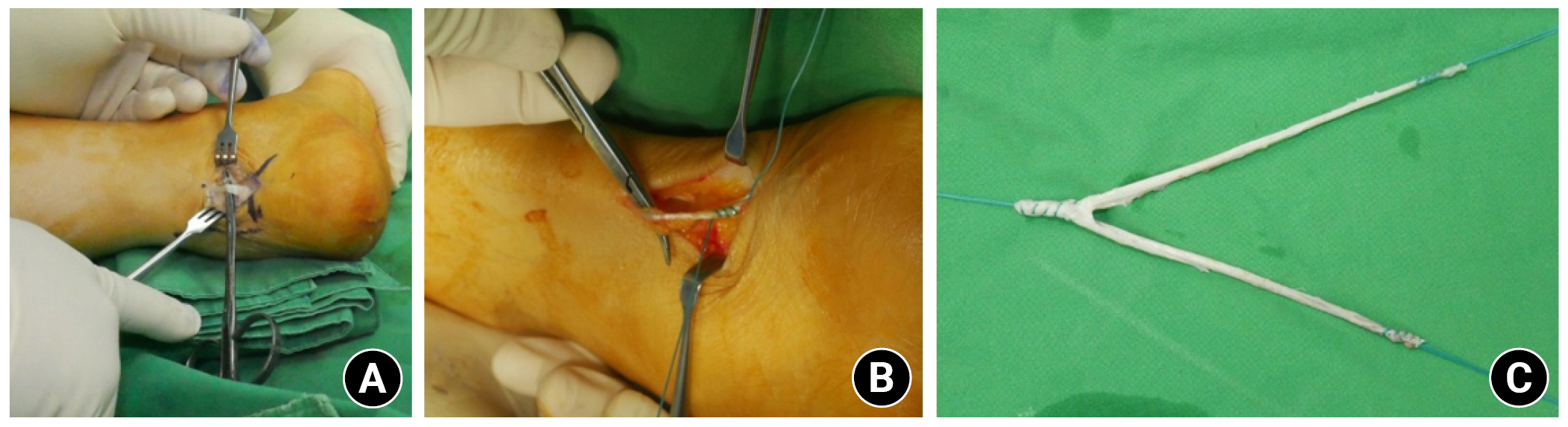
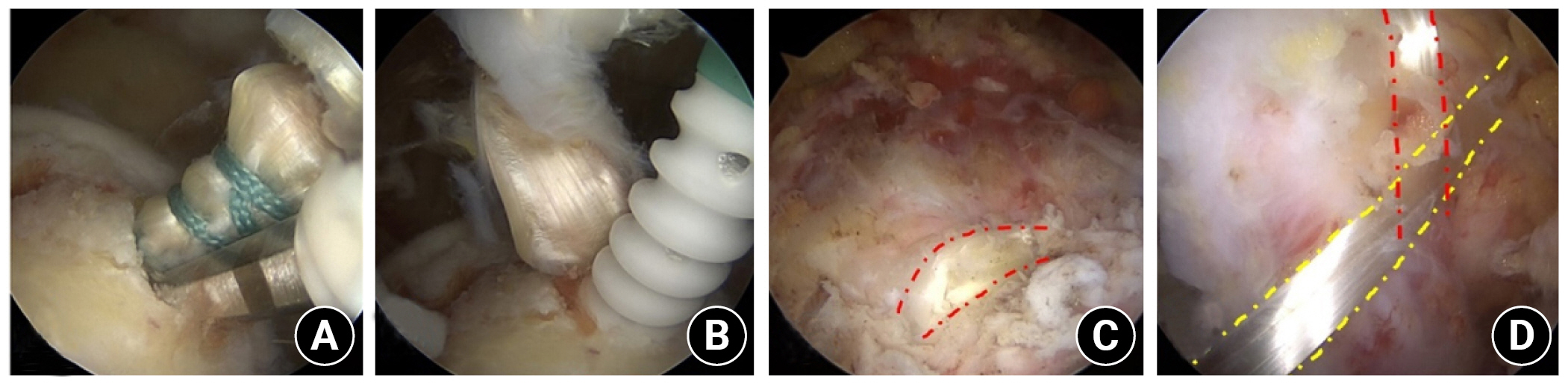
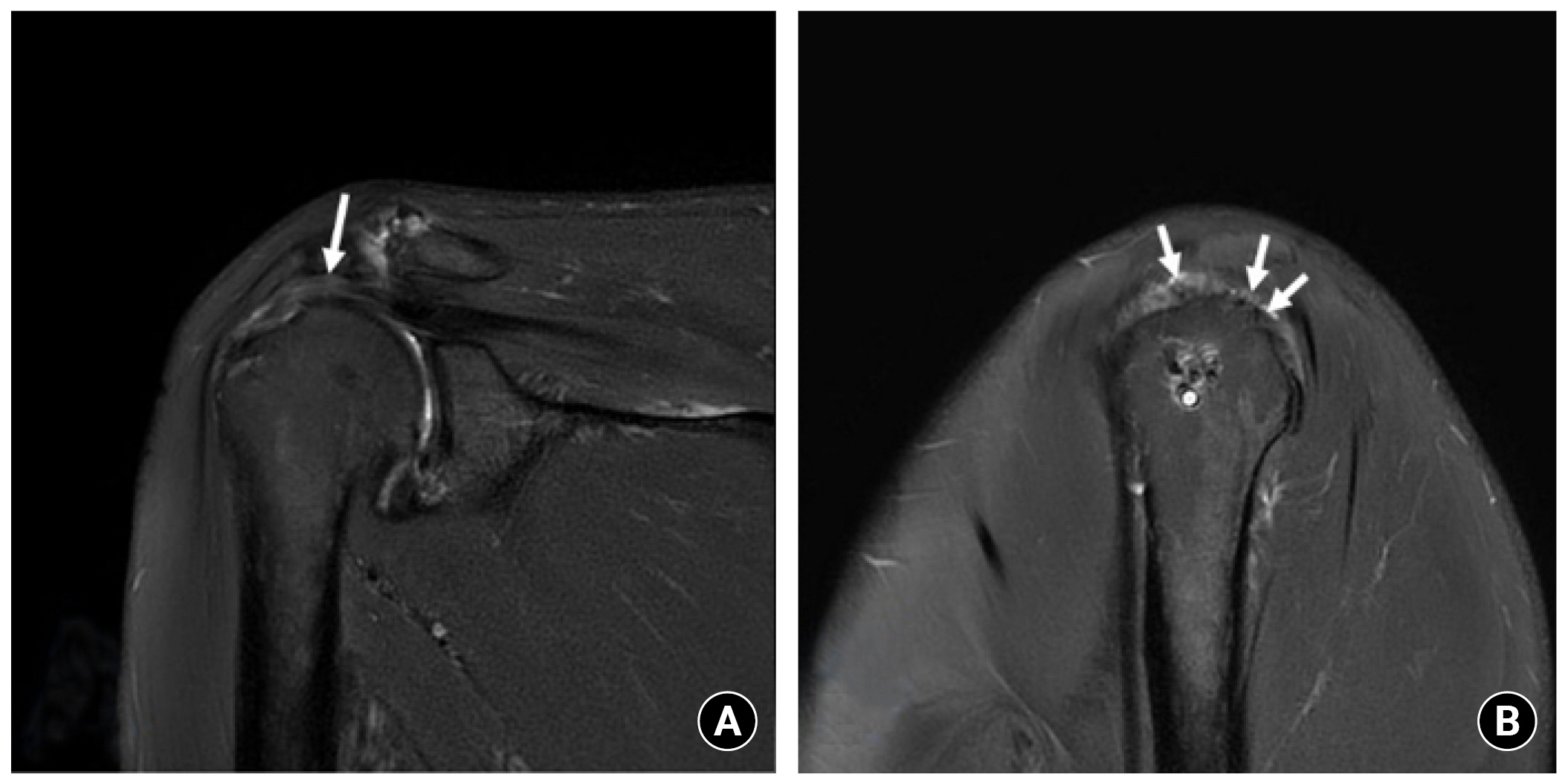
1. Yoo JC, Ahn JH, Yang JH, Koh KH, Choi SH, Yoon YC. Correlation of arthroscopic repairability of large to massive rotator cuff tears with preoperative magnetic resonance imaging scans. Arthroscopy 2009;25:573–82.


2. Warner JJ, Parsons IM 4th. Latissimus dorsi tendon transfer: a comparative analysis of primary and salvage reconstruction of massive, irreparable rotator cuff tears. J Shoulder Elbow Surg 2001;10:514–21.


3. Muench LN, Kia C, Jerliu A, et al. Clinical outcomes following biologically enhanced patch augmentation repair as a salvage procedure for revision massive rotator cuff tears. Arthroscopy 2020;36:1542–51.


4. Hersche O, Gerber C. Passive tension in the supraspinatus musculotendinous unit after long-standing rupture of its tendon: a preliminary report. J Shoulder Elbow Surg 1998;7:393–6.


5. Park SG, Shim BJ, Seok HG. How much will high tension adversely affect rotator cuff repair integrity. Arthroscopy 2019;35:2992–3000.


6. Burkhart SS, Johnson TC, Wirth MA, Athanasiou KA. Cyclic loading of transosseous rotator cuff repairs: tension overload as a possible cause of failure. Arthroscopy 1997;13:172–6.


7. Rhee SM, Youn SM, Park JH, Rhee YG. Biceps rerouting for semirigid large-to-massive rotator cuff tears. Arthroscopy 2021;37:2769–79.


8. Jones CR, Snyder SJ. Massive irreparable rotator cuff tears: a solution that bridges the gap. Sports Med Arthrosc Rev 2015;23:130–8.

9. Rhee YG, Cho NS, Parke CS. Arthroscopic rotator cuff repair using modified Mason-Allen medial row stitch: knotless versus knot-tying suture bridge technique. Am J Sports Med 2012;40:2440–7.


10. Delaney RA, Lin A, Warner JJ. Nonarthroplasty options for the management of massive and irreparable rotator cuff tears. Clin Sports Med 2012;31:727–48.


13. Mihata T, Lee TQ, Watanabe C, et al. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy 2013;29:459–70.


15. Steinhaus ME, Makhni EC, Cole BJ, Romeo AA, Verma NN. Outcomes after patch use in rotator cuff repair. Arthroscopy 2016;32:1676–90.


16. Ono Y, Dávalos Herrera DA, Woodmass JM, Boorman RS, Thornton GM, Lo IK. Graft augmentation versus bridging for large to massive rotator cuff tears: a systematic review. Arthroscopy 2017;33:673–80.


17. Bailey JR, Kim C, Alentorn-Geli E, et al. Rotator cuff matrix augmentation and interposition: a systematic review and meta-analysis. Am J Sports Med 2019;47:1496–506.


19. Maqdes A, Abarca J, Moraiti C, et al. Does preoperative subscapularis fatty muscle infiltration really matter in anterosuperior rotator cuff tears repair outcomes? A prospective multicentric study. Orthop Traumatol Surg Res 2014;100:485–8.


21. Rulewicz GJ, Beaty S, Hawkins RJ, Kissenberth MJ. Supraspinatus atrophy as a predictor of rotator cuff tear size: an MRI study utilizing the tangent sign. J Shoulder Elbow Surg 2013;22:e6–10.

22. Lippe J, Spang JT, Leger RR, Arciero RA, Mazzocca AD, Shea KP. Inter-rater agreement of the Goutallier, Patte, and Warner classification scores using preoperative magnetic resonance imaging in patients with rotator cuff tears. Arthroscopy 2012;28:154–9.


23. Davidson JF, Burkhart SS, Richards DP, Campbell SE. Use of preoperative magnetic resonance imaging to predict rotator cuff tear pattern and method of repair. Arthroscopy 2005;21:1428.

24. Reilly P, Bull AM, Amis AA, et al. Passive tension and gap formation of rotator cuff repairs. J Shoulder Elbow Surg 2004;13:664–7.


25. Gerber C, Maquieira G, Espinosa N. Latissimus dorsi transfer for the treatment of irreparable rotator cuff tears. J Bone Joint Surg Am 2006;88:113–20.


27. Zastrow RK, London DA, Parsons BO, Cagle PJ. Superior capsule reconstruction for irreparable rotator cuff tears: a systematic review. Arthroscopy 2019;35:2525–34.e1.


28. Mihata T, Lee TQ, Fukunishi K, et al. Return to sports and physical work after arthroscopic superior capsule reconstruction among patients with irreparable rotator cuff tears. Am J Sports Med 2018;46:1077–83.


29. Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary results of arthroscopic superior capsule reconstruction with dermal allograft. Arthroscopy 2018;34:93–9.


31. Senekovic V, Poberaj B, Kovacic L, et al. The biodegradable spacer as a novel treatment modality for massive rotator cuff tears: a prospective study with 5-year follow-up. Arch Orthop Trauma Surg 2017;137:95–103.


32. Mori D, Funakoshi N, Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy 2013;29:1911–21.


33. Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy 2008;24:403–9.e1.


34. Awad MA, Sparavalo S, Ma J, King JP, Wong I. Interposition graft bridging reconstruction of irreparable rotator cuff tears using acellular dermal matrix: medium-term results. Arthroscopy 2022;38:692–8.


35. Josipović M, Vlaić J, Serdar J, et al. Plantaris tendon: a novel graft for anterolateral ligament reconstruction and additional reinforcement for anterior cruciate ligament autografts in combined reconstructive procedures. Knee Surg Sports Traumatol Arthrosc 2020;28:2604–8.


36. Yammine K, Saghie S, Assi C. A meta-analysis of the surgical availability and morphology of the plantaris tendon. J Hand Surg Asian Pac Vol 2019;24:208–18.